If you’ve ever watched floating leaves as they glide down a meandering stream, you’ve probably noticed the little eddies that build up when the water bends around a sharp corner, creating turbulence in contrast to the stream’s more placid center. Look a little closer and you’ll see how complicated those disturbed flows can be, stressing and wearing away the rocks and twigs where they pass.
If you could look inside the bloodstream, you’d see the same thing at work, according to Ian Tamargo, an Emory medical student who recently completed his PhD in molecular pharmacology.
“The (blood) flow will go forward, but when it hits a curving surface, it will bounce back and spin in circles,” he says, explaining the complicated ways flow is disrupted when blood encounters a vessel wall that doesn’t run in a straight line. “In branching areas, it hits directly onto the branch point and then comes back, leading to an irregular flow pattern.”
You might picture it by thinking of a rafter trying to navigate a stretch of rapids where the water flow turns more perilous. Like those fast-moving river currents, the way our blood moves past these critical points in our vascular system can have its own kind of danger. Knowing more about those stresses and how they function could save lives through better approaches to heart disease, which the Centers for Disease Control and Prevention cites as the number one killer of adults.
It’s that idea which drives the work of Tamargo’s mentor, Hanjoong Jo, PhD, a professor of medicine and biomedical engineering at Emory who has spent more than two decades exploring new approaches to treating heart disease through understanding the complex dynamics of blood flow in this micro world.
Hanjoong Jo is professor of medicine at Emory and the Wallace H. Coulter Distinguished Chair and professor of biomedical engineering at Emory and Georgia Tech.
Beginning in the 1990s, researchers in Jo’s lab found that when blood flows around curves, such as the place where one vessel branches into two, the tiny swirls and eddies stress out the endothelium, a single layer of special cells that line the blood vessels and regulate exchanges between the bloodstream and the body’s other tissues.
This disturbed blood flow causes these endothelial cells, which are normally uniformly elongated and aligned like a “mowed down cornfield,” Tamargo says, to change their shape, to the point where they appear more like disordered cobblestones on an old street.
The increased stress not only changes the endothelial cells’ shape, but it communicates that something’s terribly wrong, sending everything into red alert mode. This leads to the thickening of vessel walls — which then leads to atherosclerosis, or the buildup of fats, cholesterol and other substances (often referred to as “plaque”) — in and on the artery walls. None of this is good news for the person who now has plaque buildup.
By contrast, in straight regions of the vessels, blood usually flows in calmer patterns, more like the center of a river than the edges. When that happens, endothelial cell responses are more likely to protect against atherosclerosis.
Atherosclerosis can be caused by many factors, including high cholesterol, diabetes, high blood pressure, a sedentary lifestyle and aging. But the blood flow stresses that promote the disease are different in that they’re not caused by external factors — and so lifestyle changes make no impact on the patient’s health.
Jo’s work is driven to figure out the best way to help patients experiencing these blood flow-caused heart blockages, by using the body’s natural systems to redirect and correct course.
“There are many cases where exercise is not a feasible solution,” he says. “By targeting flow sensitive genes, we think we will come up with a new way of treating atherosclerosis.”
Over the past decade and a half, Jo and colleagues have pioneered new techniques that let them meticulously trace hundreds of different stress pathways that lead to a greater risk of plaque buildup in the arteries.
Ian Tamargo was co-lead author of research findings published in Nature Reviews Cardiology.
Peering into their petri dishes, the investigators learned to precisely measure the effects of different types of stress on endothelial cells under laboratory conditions. Using mice, they were able to change the blood flow conditions in the left carotid artery from normal to disturbed and then watch the disturbed flow in those mice activate genes that went on to produce endothelial inflammation in as little as one week and atherosclerosis a week later. Blood flow through the right carotid artery, left undisturbed, continued normally.
“By doing this simple surgery,” Jo says, “we were able to demonstrate that flow disturbance can cause atherosclerosis in animals that have high cholesterol.”
Once they observed the how, the researchers set out to uncover the why. They found tiny bits of protein on the endothelial cells that are sensitive to stresses induced by the disturbed flow. These proteins respond by initiating the cellular signaling pathways that activate genes.
“At a microscopic level, they work almost like a windmill,” Tamargo says. “They take the mechanical force of shear stress and transmit that energy into chemical activation.”
Over time, Jo and his collaborators produced a catalog of thousands of mechanical stress-sensitive genes. “Different kinds of mechanical stress have different effects on gene expression,” Tamargo says. “And can increase or decrease the production of proteins that specifically respond to those stress patterns.”
This figure, illustrating flow-induced reprogramming of endothelial cells in atherosclerosis, was featured in a Nature Reviews Cardiology paper published in May. Ian Tamargo was a co-lead author along with Kyung In Baek, postdoctoral fellow in the Jo Lab. Hanjoong Jo was the corresponding author. Check out their full findings here: https://www.nature.com/articles/s41569-023-00883-1.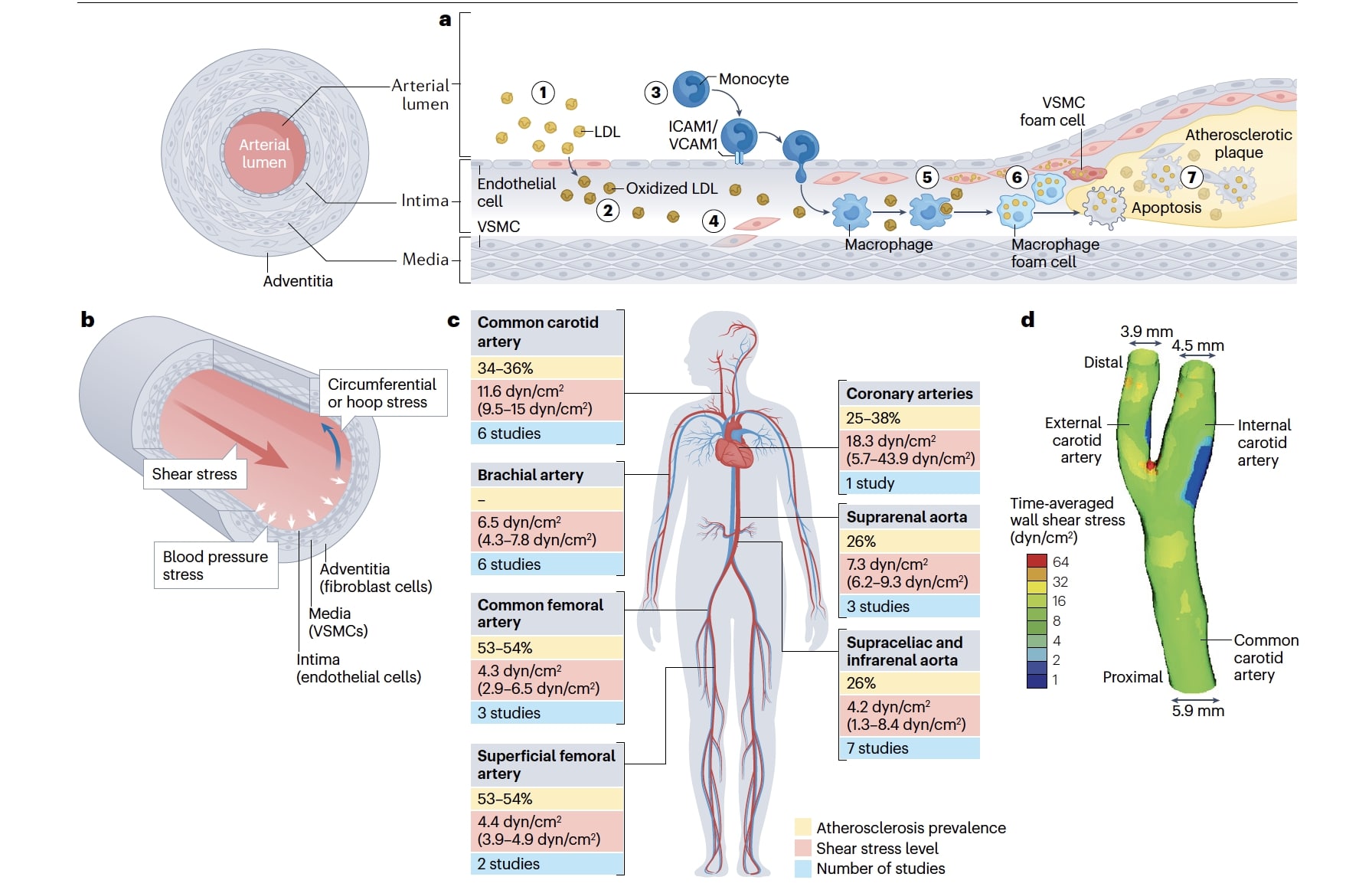
What’s next for Jo, Tamargo and their colleagues? They’ve been developing new drugs and gene therapies to manipulate the genes and proteins (also called Flow-Kines) that respond adversely to the stress of blood flow at the points that branch or curve in people’s arteries — that is, where plaque buildup “always occurs,” Jo points out.
The hope is that eventually they may be able to come up with promising new therapies, such as alternatives to lipid reducers to reduce the risk of heart attacks and strokes. Jo was encouraged by a 2017 trial of the anti-inflammatory drug canakinumab, which showed that reducing vascular inflammation could play an independent role in reducing coronary events.
As someone who sits at the intersection of bioengineering and medicine in such a unique way, Jo says he appreciates the ability to work collaboratively across different disciplines and conduct research that uses “engineering approaches to solve clinical problems.”
“I get up every day with the idea that (this work) could do something very important for our patients,” he says. “There are millions and millions of people who are dying of these diseases, and those patients don’t wait. That gets me excited about developing new therapeutics.”