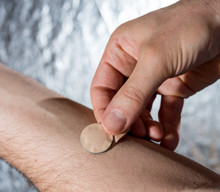
A new clinical study at Emory University is testing whether microneedle patches applied to the skin may be a safe and effective alternative to conventional flu shots. The study is currently underway and enrolling volunteers.
The patches, which are about the size of a quarter, contain very tiny (thin and short) needles, called microneedles, which are barely visible to the eye. As the patch is applied to the skin, the microneedles penetrate the upper layers of the skin and deliver the vaccine.
"We are excited to conduct this important trial at the Hope Clinic. If the patches containing flu antigens are proven safe and immunogenic, they might be a more convenient alternative to regular flu shots," says Nadine Rouphael, MD, assistant professor of medicine at Emory School of Medicine and principal investigator of the clinical study.
Researchers plan to enroll up to 100 participants in the clinical study conducted at the Hope Clinic of the Emory Vaccine Center. Healthy adults between ages 18 and 49 who did not receive a flu shot in the 2014-2015 season will receive either one dose of the regular flu shot or one patch containing either the flu vaccine or containing placebo.
The flu vaccine used in the study is the same as the FDA-approved flu vaccine from last season. The purpose of the study is to assess the safety of the microneedle patch, how the body's immune system responds to the vaccine delivered through a patch, and participants' opinions about using the patch. Participants will receive follow up, including blood work, during six visits over six months.
Researchers at Georgia Tech, led by Mark Prausnitz, PhD, Regents Professor of Chemical and Biomolecular Engineering, and collaborators at Emory University School of Medicine, have been developing the patches in a project funded by the National Institutes of Health. This is the first time the patches have been tested using actual flu vaccine.
For more information about the study, contact the Hope Clinic by phone at 404-712-1371 or by email at gosinsk@emory.edu.
