According to the American Heart Association, atrial fibrillation (AFib) is a quivering or irregular heartbeat (arrhythmia) that can lead to blood clots, stroke, heart failure and other heart-related complications in the nearly 2.7 million Americans it affects. The most common treatment to reduce stroke risk in patients with AFib is blood-thinning warfarin medication.
"The WATCHMAN device is an important advancement in stroke management for non-valvular AFib patients who are seeking alternatives to warfarin," says cardiologist David DeLurgio, MD, professor of medicine, Emory University School of Medicine and director of electrophysiology at Emory Saint Joseph's Hospital.
DeLurgio and the Emory team have been implanting the WATCHMAN device for the last seven years in clinical trials testing the new technology. He recently implanted the region’s first device since FDA approval was granted in March.
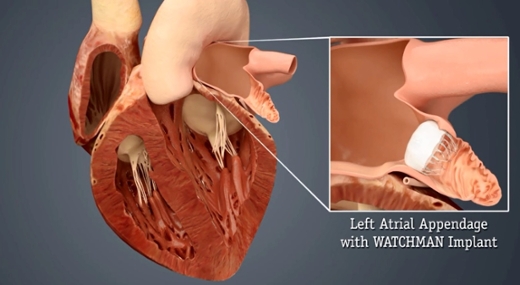
During the minimally invasive procedure, the WATCHMAN implant is delivered to the heart via a catheter, closing off an area of the heart called the left atrial appendage (LAA). The LAA is a thin, sack-like appendix attached to the heart and is believed to be the source of the majority of stroke-causing blood clots in people with non-valvular AFib.
"By closing the LAA and keeping these harmful blood clots from entering the blood stream, the risk of stroke may be reduced and, over time, patients could be freed from the challenges of long-term warfarin therapy," says DeLurgio.
Despite its proven efficacy, long-term warfarin medication is not well tolerated by some patients and carries a significant risk for bleeding complications. Nearly half of AFib patients eligible for warfarin are currently untreated due to tolerance and adherence issues.
The WATCHMAN Implant, developed by Boston Scientific, has been approved in Europe since 2005. It has been implanted in more than 10,000 patients and is approved in more than 70 countries around the world.
To learn more about arrhythmia and heart rhythm management services at Emory, please visit: www.emoryhealthcare.org/arrhythmia.
For more information on the WATCHMAN implant, please visit: watchmanimplant.com.