A new multi-center, FDA-approved clinical trial will study a new stent graft designed as a minimally invasive option for patients with juxtarenal (JAA) and pararenal (PAA) aortic aneurysms.
The trial, sponsored by Endologix, Inc., will evaluate the safety and efficacy of the Ventana™ Fenestrated System for these patients. Emory University is the only site currently enrolling patients in Georgia.
Emory School of Medicine vascular surgeon and associate professor of surgery Ravi Veeraswamy, MD, is the local principal investigator of the study.
Early clinical experience outside of the United States with the device has been promising. Until now, there has not been an “off the shelf” graft that could treat complex aneurysms that involve the renal arteries.
Previously, surgeons had to burn holes in existing grafts or have customized devices made for each patient, involving time, labor and expense. Open repair for patients with JAA or PAA – who represent approximately 20 percent of abdominal aortic aneurysm patients – is an invasive procedure that can result in significant blood loss, complication rates and long hospital stays.
Veeraswamy and his team are hopeful this new device will permit a less invasive treatment to be extended to a much larger group of patients.
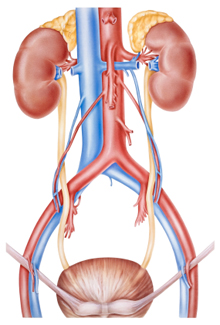
The distinguishing characteristics of the Ventana system include a main column intended to protect the aorta and exclude the aneurysm from blood flow, and two branched renal stent grafts that are inserted through the main device and into the renal arteries to maintain blood flow to the kidneys.
To learn more about the study, call 404-778-3191 or email rveeras@emory.edu.
