How can scientists probe into the human brain, whose development is both intricate and inaccessible? An approach gaining popularity at Emory and around the world is to grow brain-like structures in the laboratory, using human cells. These structures are called brain organoids.
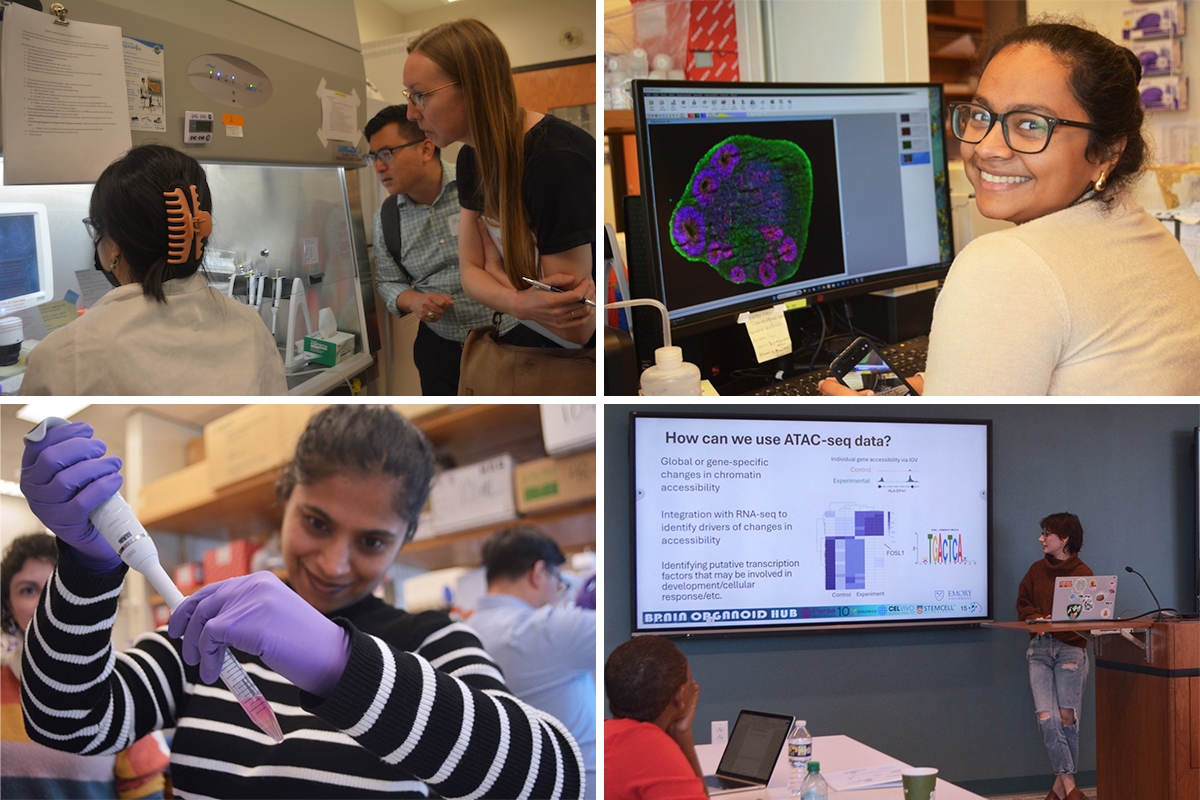
In mid-October, Emory’s Department of Human Genetics held a workshop aimed at teaching others the latest techniques for culturing and probing brain organoids. Ten scientists from the United States, Spain, Japan and Australia spent five days learning how to grow brain organoids and how to visualize and analyze the cells inside them.
The workshop was held by the department’s Brain Organoid Hub, founded in 2022 by the heads of three laboratories: Jimena Andersen, Fikri Birey and Steven Sloan. The trio trained together as postdocs in the lab of Sergiu Pasca at Stanford, where they developed many of the techniques and protocols they are now teaching to others.
In founding the Hub, the trio wanted to tackle challenges facing the relatively new brain organoid field, such as reproducibility and quality control. They also wanted to engage in education and outreach. The result is like America’s Test Kitchen — for a set of very complex laboratory procedures.
“With the Hub, we have the opportunity to build something bigger than we could in each of our individual labs,” says Steven Sloan, MD, PhD, associate professor of human genetics at Emory University School of Medicine.
Patient-derived cells
Brain organoids are generated from induced pluripotent stem cells or iPSCs, which have the potential to become any type of cell in the body. iPSCs come from skin or blood cells that are reprogrammed in the laboratory, in a process whose discovery won part of a 2012 Nobel Prize.
Under finely tuned culture conditions, iPSCs can be guided to develop into brain or spinal cord cells. Scientists direct those conditions so that the cells organize themselves into structures that resemble the layers and cell types found in regions of the brain, such as the forebrain, striatum or hypothalamus.
Unlike conventional cell cultures grown in flat layers, organoids develop in three dimensions. However, they lack components of a full brain, such as blood vessels and separate lobes. (Several other types of organoids are studied at Emory as well, such as intestinal, airway or cardiac.)
Because brain organoids can be created from cells donated by individuals with neurodevelopmental or neurodegenerative disorders, they provide unique opportunities to study these conditions. Starting with cells derived from patients with autism spectrum disorders, schizophrenia or epilepsy, researchers can track the appearance of pathophysiological markers and test potential therapies.
Organoids can also help researchers answer fundamental questions about human brain development, such as where specific cell populations come from and how they migrate to their destinations.
“Experimental approaches using brain organoids should be seen as complimentary to approaches using mice or other model organisms,” Birey says. “Organoids do offer some advantages in capturing human-specific aspects of brain development, as well as scalability and accessibility.”
The Andersen lab is taking a next step, combining brain with spinal cord and muscle organoids. She and her colleagues are using the ensuing “assembloids” to study ALS (amyotrophic lateral sclerosis).
“While we know that motor neurons progressively degenerate in ALS, other cells interacting with them can contribute to or exacerbate this degeneration,” Andersen says. “Assembloids provide an opportunity to study these interactions in a dish.”
Core facilities vs. the Brain Organoid Hub
Emory and other research universities have core facilities that provide well-defined services to researchers, such as DNA sequencing or electron microscopy. The Hub is more about collaboration, and is not serving up organoids to order on a fee-for-service basis, although its members work closely with the Emory Stem Cell and Organoids Core.
Some of the workshop participants came from core facilities, such as the Human Neuron Core at Boston Children’s Hospital. Others were in or had just finished graduate school, and wanted to learn techniques that could help them start a new project.
“I think this will be a great resource,” says Marcus Li, PhD, a workshop participant and group lead for neuroscience at University of California San Francisco’s Laboratory for Genomics Research. “This workshop gives me the innovative and necessary tools, so I can go back and establish that capability in our labs. It could bloom into a long-term collaboration.”
Sponsors for the workshop provided supplies and support so that participants could stay in Atlanta for the week. They were: Stem Cell Technologies, Parse Biosciences, Cell Vivo, 10x Genomics and the Department of Human Genetics.