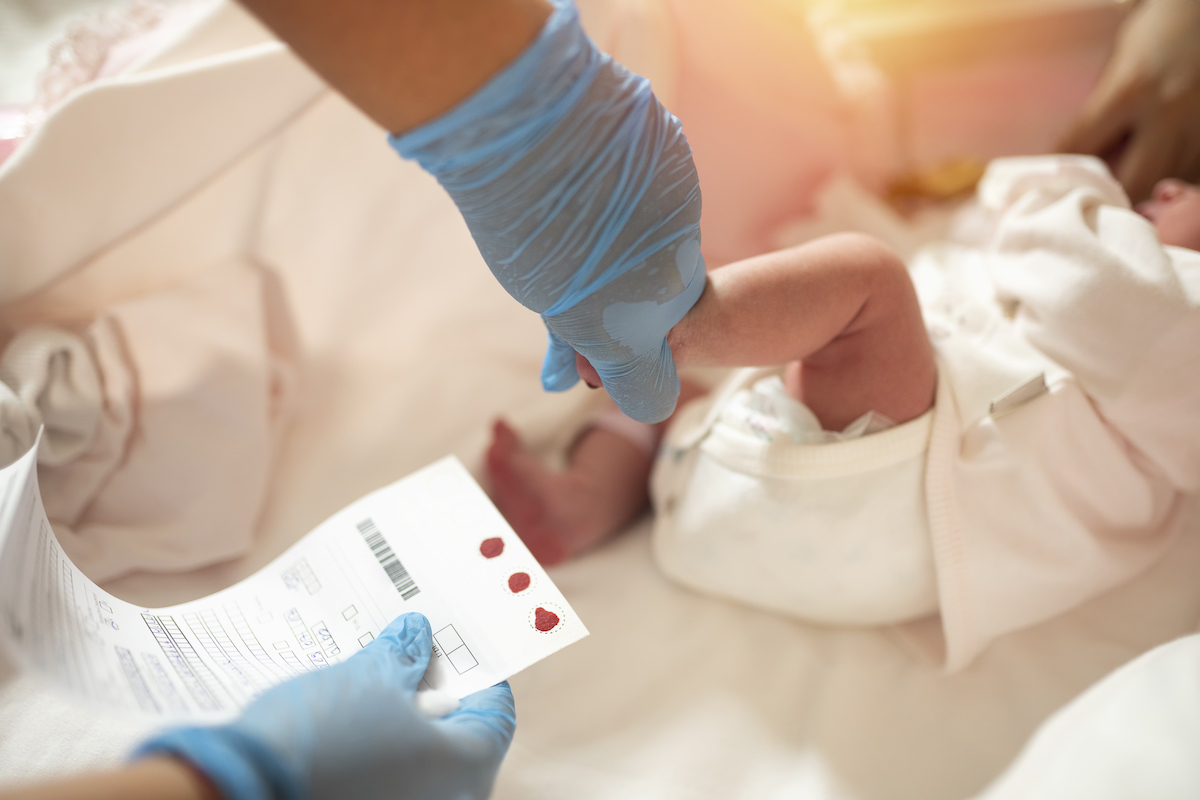
Emory University’s Medical Nutrition Therapy for Prevention (MNT4P) program has been awarded $2 million to assess and optimize the effectiveness of newborn screening programs in the Southeast United States over the next four years.
Nationwide, more than 3 million newborn babies undergo testing each year by having blood spots from heel pricks analyzed for biochemical abnormalities. This process — called newborn screening — is designed to quickly identify infants with rare disorders such as phenylketonuria or cystic fibrosis, which could lead to significant morbidity, mortality or intellectual disability if left untreated.
Funding from a cooperative agreement with the U.S. Health Resources and Services Administration (HRSA) will support the Southeast Integrative Newborn Screening – Long-Term Follow-Up Consortium, the only nationally funded multi-state consortium. This consortium includes Georgia, Florida, Louisiana and Mississippi health departments and partners with other states in the Southeastern Regional Genetics Group (SERGG). The collaboration will strengthen the newborn screening system through SERGG’s established framework and partnership.

Rani H. Singh, PhD, RDN, LD, is principal investigator of the corsortium.
“Our goals for the consortium are to enhance the efficiency and timeliness of newborn screening sample collection, testing and reporting; examine equity and disparities; and improve short- and long-term newborn screening follow-up, all while meaningfully engaging and partnering with the providers and families at every level of the newborn screening system to improve patient outcomes,” Singh says.
The consortium will use a multifaceted approach to improve newborn screening programs, focusing on two areas:
- Ensuring initial newborn screening specimens are collected within the appropriate timeframe, no later than 48 hours after birth. A robust and secure data exchange infrastructure will be implemented regionally and nationally.
- Identifying and developing common long-term follow-up data elements for specific disorders, aiming to increase access to treatment and follow-up activity for patients and their families with conditions identified through newborn screening.
Singh has previously served as a principal investigator for HRSA-funded newborn screening initiatives supporting the Southeast Regional Newborn Screening & Genetics Collaborative (2006-16) and the Southeast Regional Genetics Network (2017-24).