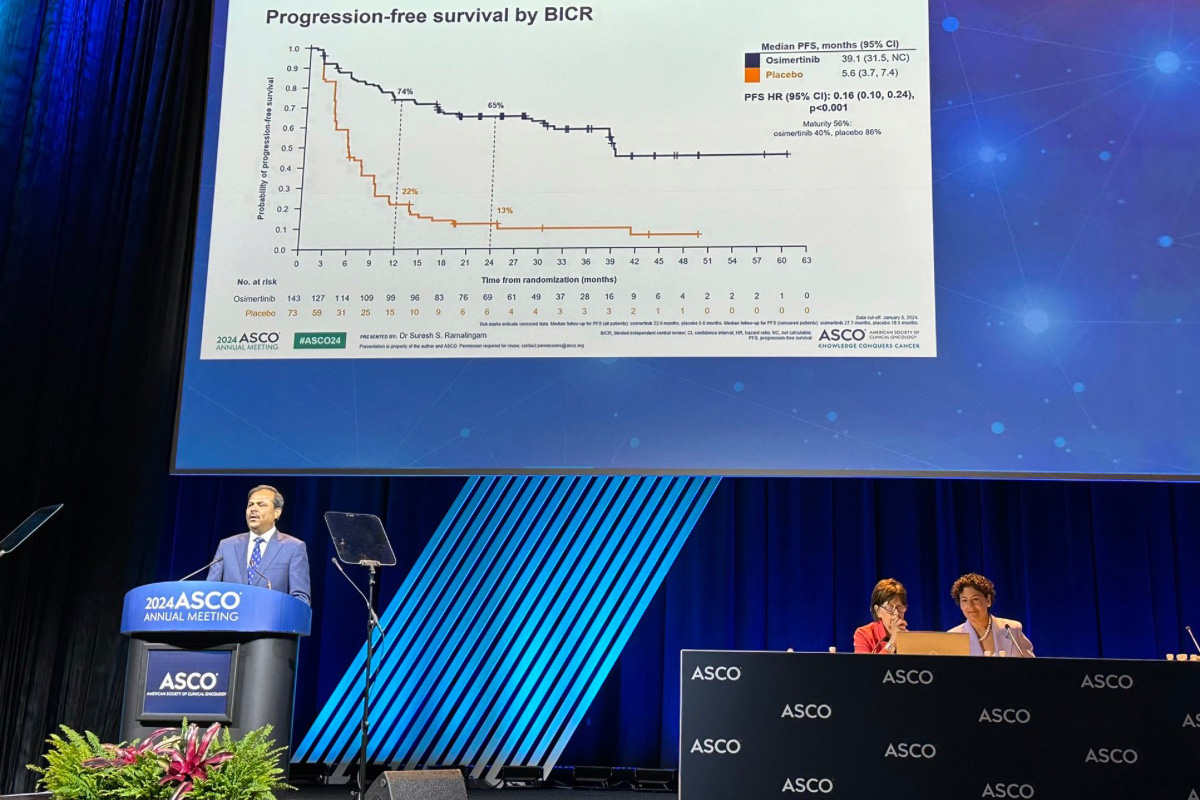
In a recent study led by Suresh Ramalingam, MD, executive director of Winship Cancer Institute of Emory University, a new drug has shown remarkable promise in treating patients with a specific type of advanced lung cancer. Osimertinib, produced by AstraZeneca under the brand name Tagrisso, significantly extends the time patients live without their cancer worsening, offering the first effective therapy and new hope for those with stage III non-small cell lung cancer (NSCLC) who have mutations in the epidermal growth factor receptor (EGFR), a protein that controls cell division and survival.
Key findings

Patients participating in the trial had already undergone standard treatments, including chemotherapy and radiation, without their cancer progressing further. Participants were randomly assigned to receive either osimertinib or a placebo.
The results were compelling: patients taking osimertinib experienced a median progression-free survival of 39.1 months, compared to just 5.6 months for those on the placebo. This means that osimertinib reduced the risk of disease progression or death by an impressive 84%.
Broader impacts
Osimertinib is the first and only EGFR inhibitor to show such a benefit in the stage III setting, extending progression-free survival by more than three years. The study demonstrated a clinically meaningful benefit across various patient subgroups, including differences in sex, race, type of EGFR mutation, age, smoking history and prior treatments.
While overall survival data are still maturing, early results indicate a favorable trend for osimertinib. The trial will continue to monitor overall survival as a secondary endpoint.
“The impressive progression-free survival results from the LAURA Phase III trial represent a major breakthrough for patients with stage III EGFR-mutated lung cancer for whom no targeted treatments are available,” says Ramalingam. “Osimertinib delayed the risk of disease progression or death by an unprecedented 84% and should become the new standard of care for patients in this setting based on these data.”
Safety and approval
Regarding safety, 35% of patients on osimertinib experienced serious side effects, compared to 12% in the placebo group. The most common issue was radiation pneumonitis, an inflammation of the lungs caused by radiation therapy, affecting nearly half of the patients in both groups. Importantly, no new safety concerns were identified.
Osimertinib is already approved as a monotherapy in more than 100 countries, including the U.S., EU, China and Japan, for various stages and types of EGFR-mutated NSCLC. These latest findings further solidify its role as a crucial treatment option.
Conclusion
The positive results from the LAURA Phase III trial underscore the importance of early testing and diagnosis in lung cancer, which remains the leading cause of cancer death worldwide, accounting for about one-fifth of all cancer deaths. Each year, an estimated 2.4 million people are diagnosed with lung cancer globally, with non-small cell lung cancer (NSCLC) being the most common form.
EGFR mutations are found in a significant subset of NSCLC patients, particularly in Asia, making targeted therapies like osimertinib vital in treatment.
“Tagrisso extended progression-free survival by more than three years in this potentially curative setting, reinforcing the need to test and diagnose patients early. These practice-changing data cement the powerful impact Tagrisso can make as backbone therapy in EGFR-mutated lung cancer,” says Susan Galbraith, executive vice president of oncology R&D at AstraZeneca, which funded the study.
For more information about the clinical trial, details can be found under the identifier NCT03521154.