Emory Vaccine Center researchers say they have identified the Achilles heel of SARS-CoV-2, the virus that causes COVID-19.
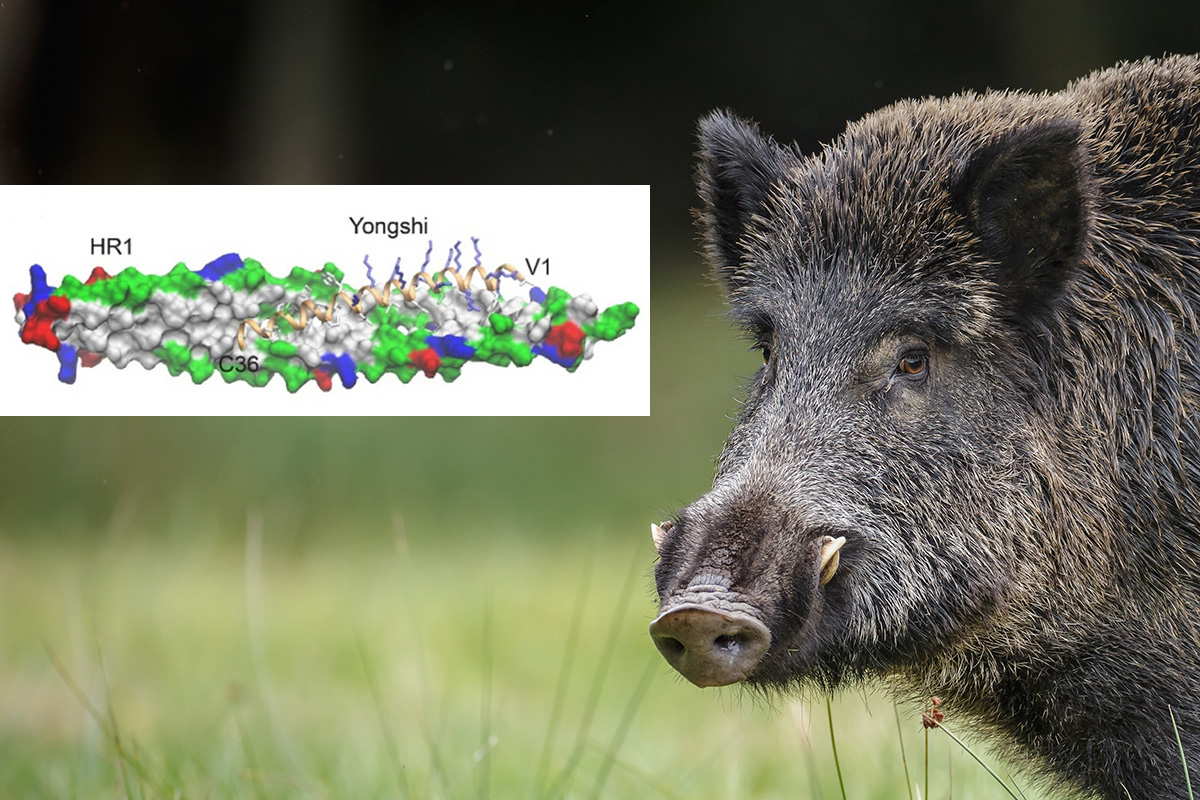
Part of the viral fusion machinery seems to stay the same among several viral variants, and this vulnerable machinery is what the antiviral peptide Yongshi targets. A peptide is a small protein or fragment of a protein.
Derived from a natural antiviral peptide produced by wild boar, Yongshi (meaning “warrior” in Mandarin) can prevent SARS-CoV-2 from infecting host cells. The scientists are now studying how to adapt the peptide for clinical use.
The results were published in Scientific Reports.
“Yongshi throws a wrench into the machine that allows SARS-CoV-2 to enter the host cell,” says senior author Joshy Jacob, PhD, professor of microbiology and immunology at Emory University School of Medicine. Jacob’s Vaccine Center lab is based at Emory National Primate Research Center.
An advantage for Yongshi is that it appears to be active against all known SARS-CoV-2 variants, including the recent XBB.1.16. It is also active against the related viruses SARS-CoV-1 and MERS. For comparison, monoclonal antibodies developed against SARS-CoV-2 variants have often lost activity as the virus continues to evolve and new variants emerge.
“This is important because drug makers and the public in general has to catch up whenever a new viral variant takes the stage,” Jacob says. “A peptide-based antiviral approach might allow us to stay ahead.”
The origin of Yongshi was in cathelicidin peptides, which are produced by mammals, birds, amphibians and fish and have long been studied for their antimicrobial properties.
Working in Jacob’s lab, former graduate student Troy von Beck tested cathelicidin peptides from several types of animals, including bats, pangolins, humans, whales, snakes, cats, koalas and wallabies. He settled on one from wild boar, which was the most active, and then modified it to reduce toxicity against human cells. Pigs are not susceptible to SARS-CoV2, and it is unclear whether their cathelicidin peptides may be partly responsible.
To understand the mechanism of Yongshi’s antiviral activity, the Emory researchers collaborated with computational protein-modeling experts Jeffrey Skolnick, PhD, and Mu Gao, PhD, at Georgia Tech.
In a computer simulation, they identified interactions between Yongshi and part of the SARS-CoV-2 spike protein known as the “helical bundle.” Further experiments with the Emory-based labs of Mehul S. Suthar, PhD, and Renhao Li, PhD, supported this model. Previously discovered helical bundle peptide inhibitors are expected to be cross-reactive with SARS-CoV-2 antibodies and may be ineffective in vaccinated or convalescent individuals.
Jacob’s lab has a track record of finding antiviral peptides from the natural world. In collaboration with Indian herpetologists, his team isolated an anti-flu peptide from frog skin secretions. They later found another peptide from frogs against Zika and dengue viruses.
Developing antimicrobial peptides into effective drugs has been a challenge, partly because enzymes in the body can break them down.
Jacob says that further modifications to the peptide are necessary to generate a therapeutically viable product. This may include chemical modification, which could both reduce the necessary dose of peptide and improve its stability following administration. Also, Jacob suggests that mRNA technology used in vaccines against SARS-CoV-2 vaccines could be a way to deliver Yongshi to patients long-term, either as a single-dose therapeutic following infection or as a prophylactic prior to infection.
The research was supported by an Imagine, Innovate and Impact research award from Emory University School of Medicine.