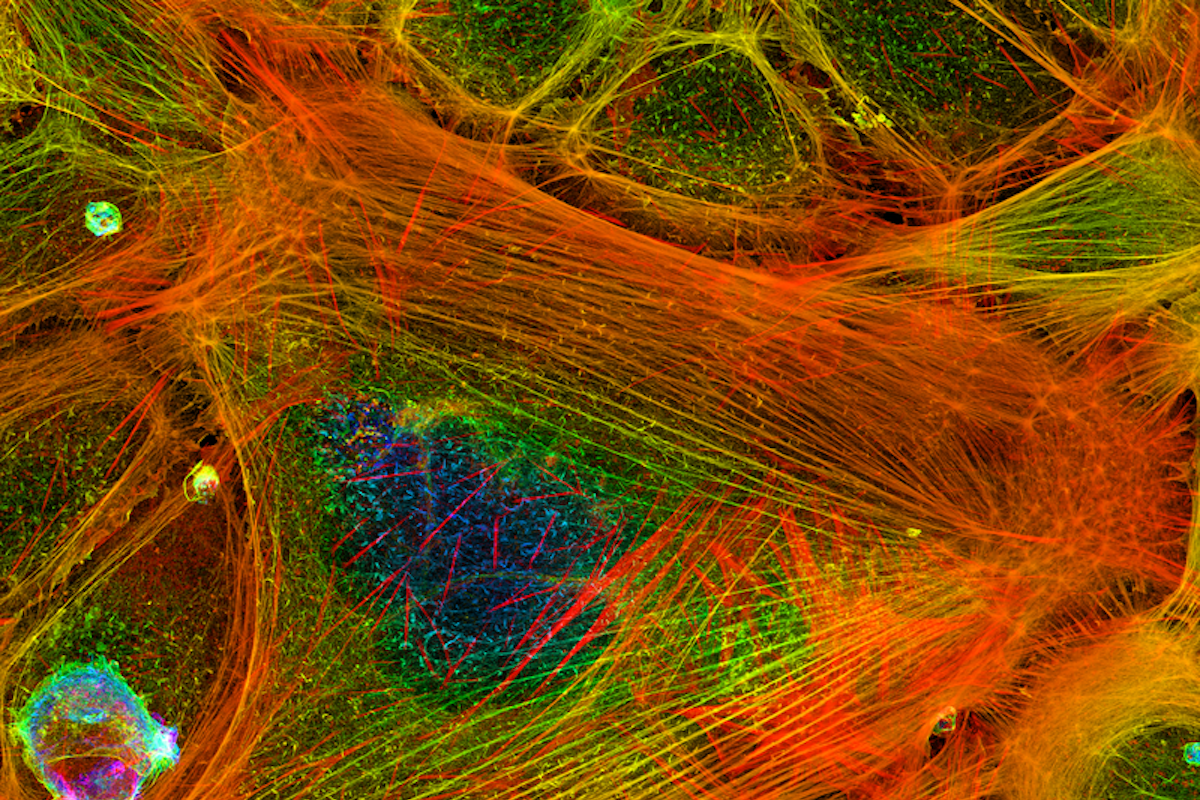
A single human cell teems with as many 100,000 different proteins. Actin is one of the most abundant and essential of them all. This protein forms into filaments that help make up the skeleton of cells, giving them shape. And as the actin filaments elongate, they work like muscles, pushing against the inner membrane of a cell to move it forward.
Three other proteins are known to drive the activities of actin. One class of protein assembles individual actin molecules into actin filaments, another causes the filaments to stop growing and a third disassembles filaments.
Biophysicists at Emory University, however, have discovered an even more complex and nuanced view of how these three proteins together influence actin dynamics. Nature Communications published the findings, showing how these proteins sometimes shift from solo or duet acts to perform as a trio, allowing them to fine-tune the activity of actin filaments.
The discovery opens another window onto the dynamics of cellular movement, which is key to processes ranging from stem-cell differentiation and wound healing to the development of diseases such as cancer.
“We found that while these three proteins do one thing when working on their own, they do a completely different thing when the other two proteins join them,” says Shashank Shekhar, Emory assistant professor of physics and cell biology, and senior author of the study. “It gets really complex, very fast.”
“No one had looked at all of these proteins interacting at once on actin,” adds Heidi Ulrichs, co-first author of the study and an Emory PhD candidate in biochemistry, cell and developmental biology. “Our paper is the first report of all three of them occupying the same barbed end of an actin filament.”
Ulrichs worked closely on the project with Ignas Gaska, a postdoctoral fellow in the Shekkhar lab who is co-first author of the paper.
Building on previous research
Research into how proteins act individually on actin is relatively well-characterized.
A polymerase protein, such as formin, drives elongation of actin. Formin positions itself at the end of an actin filament, grabs onto free-floating actin molecules and stacks them up one by one to keep growing the end.
Depolymerase proteins, such as twinfilin, are another class of proteins that influence actin. Twinfilin works like a lint roller, binding to the end of a filament and peeling away one molecule at a time. Twinfilin can repeat the process to disassemble the actin filament entirely.
Proteins known as cappers can stop the elongation and disassembling of the filaments. A capper attaches to the end of an actin filament and covers it like a hat, blocking activity by the other proteins.
This knowledge was built up by isolating one protein at a time to study how it influences actin. More recent studies have also shown simultaneous interactions between twinfilin and capping proteins.
A new approach using advanced technology
For the current study, the researchers wanted to explore whether formin, twinfilin and the capping protein could all three act simultaneously on actin.
“An actin filament end is really tiny, just five nanometers across,” Shekhar explains. “One thought was that there just isn’t enough real estate available for three proteins to work on a single actin filament at once.”
The Shekhar Lab is one of only a handful in the world using the highly specialized technique of microfluidics-assisted total internal reflection fluorescence microscopy (mf-TIRF) to study how the actin cytoskeleton remodels itself.
Cells are packed with thousands of proteins moving around, performing different functions, making it impossible to track all of them. Researchers must isolate the proteins of interest and study them outside of a cellular system, by introducing them to a microfluidic system on a microscope slide.
The mf-TIRF technology allows the Shekhar Lab to attach fluorescent orbs to single protein molecules so that researchers can better observe what these molecules are doing through a microscope.
In experiments, the researchers tagged molecules of actin, formin, twinfilin and the capping protein with four different colors that emitted fluorescent light. They then introduced actin to the microfluidic system and added the other proteins one at a time.
Establishing a new paradigm
The results startled them.
When twinfilin, the protein that breaks apart an actin filament, was added in the presence of both formin and the capping protein, twinfilin actually worked to speed up the process of filament elongation.
“That’s counterintuitive, which is cool,” Ulrichs says. “Doing science you get surprised all the time.”
Twinfilin alone could not join formin on the end of the actin filament. However, when the capping protein was also present, all three could simultaneously work together on the tiny surface of the actin filament.
Shekhar compares the effects of all three proteins working together to a knob that allows for more precise control of a process.
“Our findings establish a new paradigm in which the three proteins work in concert to fine-tune how fast or slowly actin filaments are formed,” he says.
The dynamics of how the three proteins interact with actin is fundamental to teasing apart the complex mysteries of how cells function normally and what happens when something goes wrong.
“We’re building up knowledge, step by step, study by study, on the dynamics of what’s happening inside of a cell,” Ulrichs says.
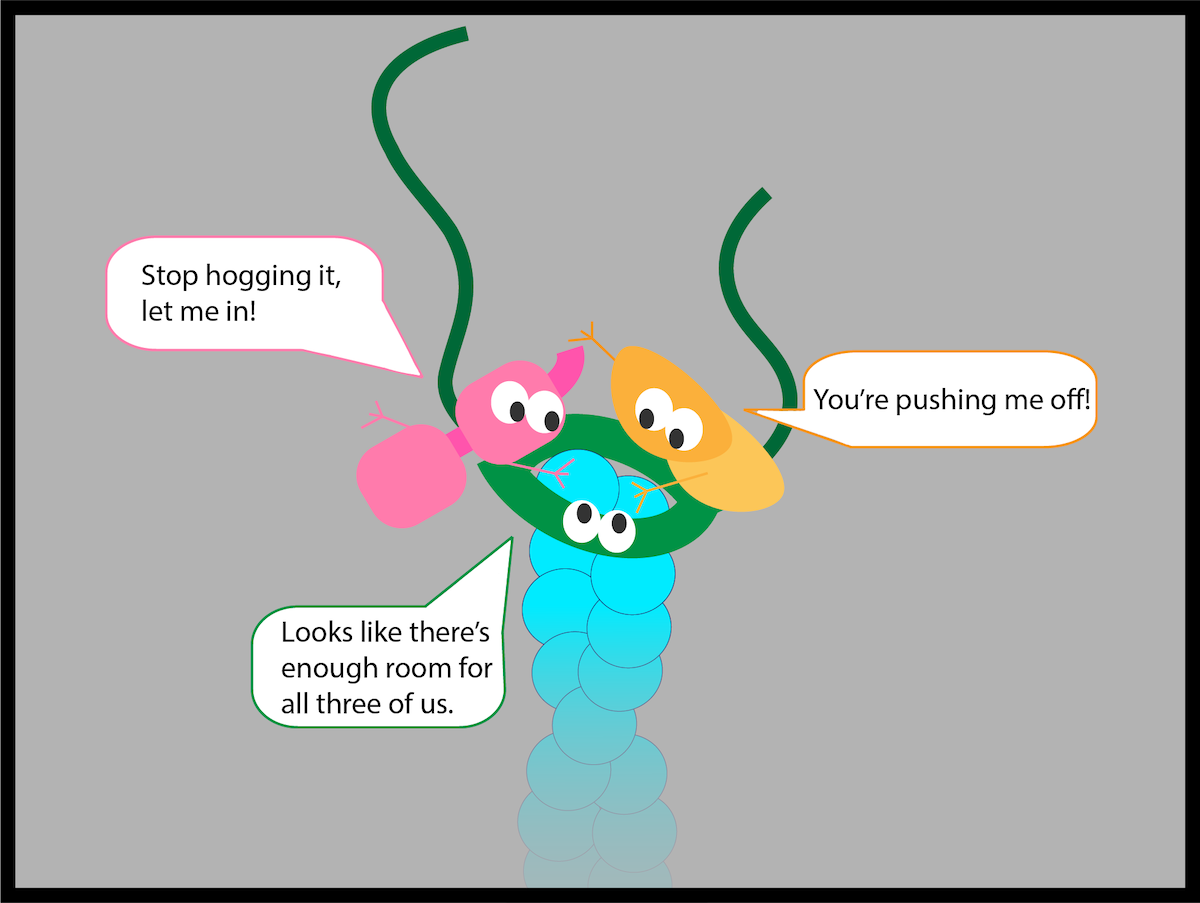
Graduate student Heidi Ulrichs, co-first author of the paper, created this cartoon to illustrate previous theories that three enzymes could not simultaneously occupy the end of an actin filament. Her drawing shows an actin filament (in blue) with the enzymes, in pink, gold and green, engaged in a kind of “sibling rivalry.” The Emory physicists discovered that, in fact, the three enzymes could simultaneously work together on the end of an actin filament.