Even after three years since the emergence of COVID-19, much remains unknown about how it causes severe disease, including the widespread organ damage beyond just the lungs. Increasingly, scientists are learning that organ dysfunction results from damage to the blood vessels, but why the virus causes this damage is unclear. Now a multidisciplinary team of Emory researchers has discovered what they believe is the key molecular pathway.
Results of their study, published today in Nature Communications, show that COVID-19 damages the cells lining the smallest blood vessels, choking off blood flow. These results could pave the way for new treatments to save lives at a time when hundreds of people are still dying from COVID-19 each day.
Doctors at Emory started this study in the early days of the pandemic to better understand drivers of severe COVID-19 and why adults develop severe disease more often than children. They used a so-called “multi-omics” approach, studying multiple data sets at once, to examine the biochemistry of blood from COVID-19 patients and compared it to non-COVID-19 patients, looking for clues.
“We were surprised by the little overlap between our adult and pediatric patients,” says Cheryl Maier, MD, PhD, assistant professor in the Department of Pathology and Laboratory Medicine, Emory University School of Medicine, and the study’s senior author. “Both groups had abnormalities related to clotting, but one unique pathway that stood out in the adults was related to vessel health and blood flow.”
Maier says this finding was particularly interesting given their clinical observations that blood from patients severely ill with COVID-19 was unusually viscous: think maple syrup rather than water.
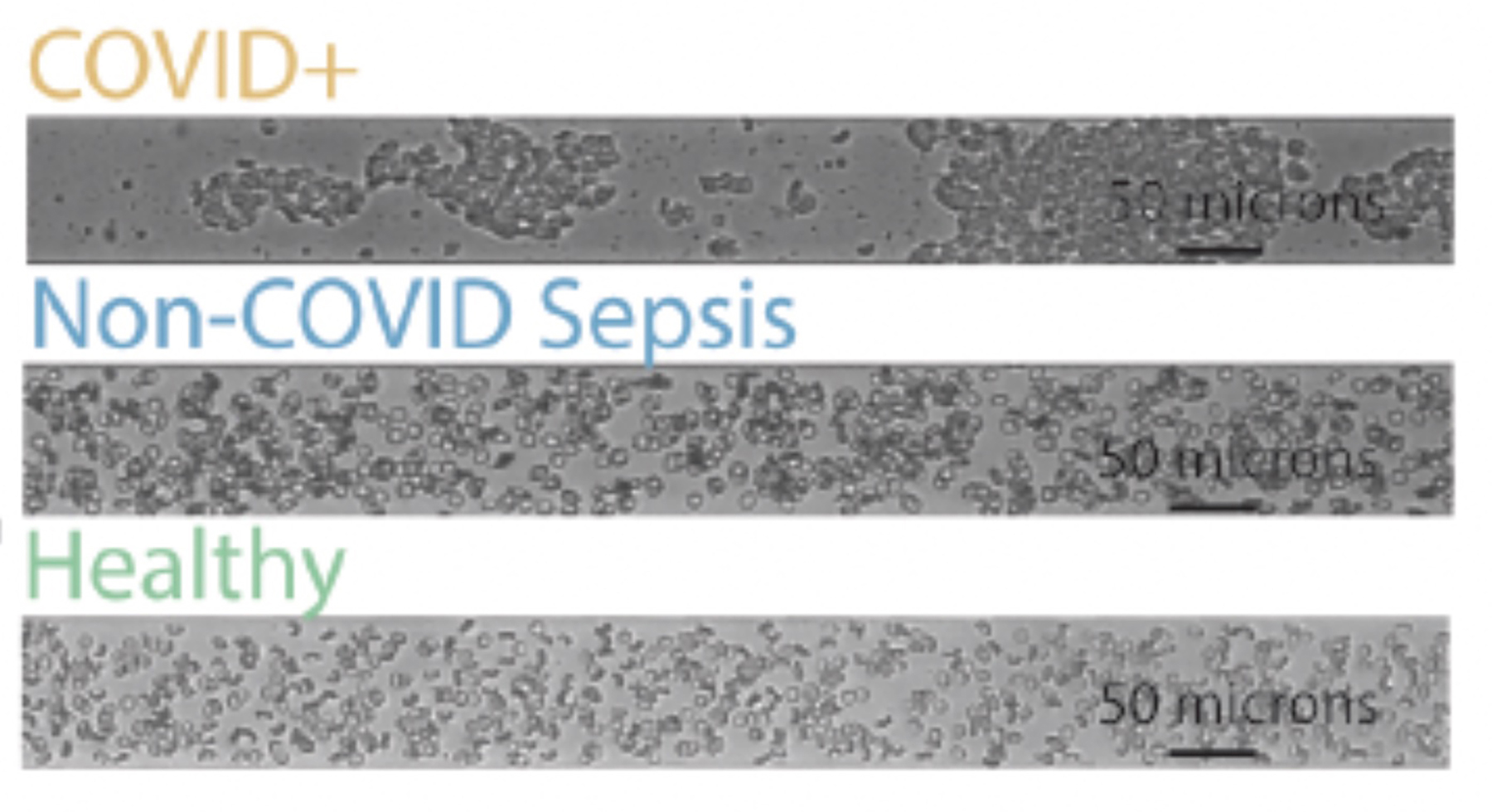
Maier worked with collaborator and co-senior author Wilbur Lam, professor in the Department of Pediatrics at Emory University and in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Institute of Technology and Emory University, to create cutting-edge models of the smallest blood vessels, expected to be the most sensitive to altered blood flow, which allowed them to visualize how blood from COVID-19 patients versus other patients might be flowing in the human body.
“Watching videos from these microfluidic devices is like seeing how COVID-19 might be affecting our blood vessels in real time,” Maier says. “These lab-made blood vessels are lined with real human vascular cells, called endothelial cells. You can put in plasma and red cells, any of the key components of blood and in different combinations, to watch how it behaves and see how the damage happens.”
Fibrinogen: A key culprit?
Since the earliest days of the pandemic, physicians have seen that a blood protein called fibrinogen was extremely elevated in patients with severe COVID-19. This protein is often elevated in other acute illnesses, but the elevations seen in the sickest COVID-19 patients were much higher. The body forms blood clots in part by cutting fibrinogen to form fibrin, a key component of clots, but fibrinogen itself is not thought to form clots and levels are not affected by anticlotting medications. But the Emory researchers found that in COVID-19 patients, the sky-high levels of fibrinogen cause red blood cells to clump together, altering blood flow and directly damaging the endothelial glycocalyx, a gelatinous protective layer lining the microvessels.
“Fibrinogen is one of the top three most abundant proteins in plasma,” Maier says. “It’s been hiding in plain sight.”
When the researchers combined plasma from COVID-19 patients with red blood cells in lab-made blood vessels, they could visualize the cellular aggregation and quantify the destruction of the endothelial cell glycocalyx. “You have these large clusters of red cells that are all stuck together,” Maier says. “Normally this wouldn’t happen. Capillaries are so narrow that red blood cells must pass through single file. But in COVID, these aggregates stick together even under flow. It’s easy to imagine how this mechanically damages the microvasculature.”
Much of the new technology was developed by study co-first author Elizabeth Iffrig, MD, PHD, a critical care fellow in Emory’s Department of Medicine. “The foundation of what we did was looking at how red blood cells would form these big globules that would gunk up the microvascular system,” Iffrig says. “Our methodology let us look at this in a dynamic process, seeing what happens to these aggregates as we mimic a true physiologic state of blood flow instead of just suspending them in a fluid and measuring how big they are. The methodology allowed us to quantify all those things simultaneously.”
Taken together, these data suggest to Maier that the fibrinogen-induced red blood cell aggregation and resulting microvascular damage could be the major pathway by which COVID causes organ damage and even death. There’s presently no medications targeting high fibrinogen in the blood. However the team has done exploratory research using therapeutic plasma exchange: removing plasma with high fibrinogen from COVID-19 patients and replacing it with donor plasma that has normal fibrinogen levels. Maier thinks her team’s discovery is critical because it provides a target that might help save lives.
Additional key members of the research team include co-first author Sam Druzak, PhD, and co-senior author Eric Ortlund, PhD, professor in the Department of Biochemistry at Emory University.