For years, the phrase “gene therapy” sounded like science fiction.
Now it’s become reality for a number of diseases as researchers refine once-experimental approaches. Emory’s Genetic Clinical Trials Center (GCTC) was designed as a hub for testing the increasing number of products aimed at genetic diseases.
“Our goal is to bring the latest treatments to our patients sooner,” says William Wilcox, MD, PhD, professor of human genetics at Emory University School of Medicine and GCTC investigator. “We work with a lot of different specialists and industry partners to bring these things to market so we can help our patients.”
As part of a clinical trial testing a gene therapy for Fabry disease, Curtis Primus travels to Atlanta every month from Wisconsin. Along with other members of his family, Primus was diagnosed with Fabry disease as a child, based on characteristic streaks in the cornea of their eyes.
Fabry disease involves a deficiency in alpha galactosidase A, an enzyme that breaks down lipids. As a result, a type of lipid called GL3 (globotriaosylceramide) builds up in the nervous system, blood vessels and kidneys, along with the eyes – hence the streaks. People with Fabry disease have an increased burden of cardiovascular disease and kidney problems. They also have impaired functioning of their sweat glands and sometimes experience episodes of pain, because of damage to the nervous system.
“It would feel like, when I’d slide a shirt on, it would feel like someone’s running sandpaper all over my body, wherever the clothing was touching,” Primus says. “Sometimes it would feel like they’re pulling my skin off with pliers.”
Enzyme replacement therapy for Fabry disease became available in 2001 in Europe and 2003 in the United States, and it was a significant advance. However, patients can describe its limitations: the effect on the body is not continuous. Some people develop antibodies to the enzyme, which reduces its effectiveness, or allergic reactions to it. That’s why gene therapies were developed and other treatments continue to be tested.
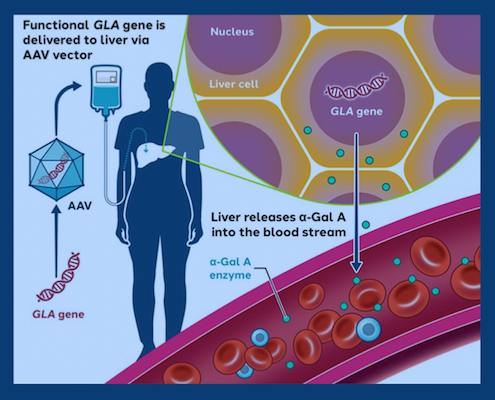
Primus is part of a clinical trial evaluating a viral vector, which is based on AAV (adeno-associated virus), a benign virus. Hundreds of clinical trials are underway involving AAV vectors, and a few products based on AAV vectors have been FDA approved, including gene therapies for spinal muscular atrophy and hemophilia B.
AAV acts as a vehicle for delivering a circle of DNA encoding the missing enzyme to the liver. The circles stay in the liver for years, but they do not become part of chromosomes and are not transmitted to children.
“If the viral vector makes enough enzyme, it will leak some outside of liver cells and travel to the rest of the body,” Wilcox says. “How much that occurs remains to be seen. Can it make enough to treat the whole body, and for how long? That’s why we’re doing the clinical trials.”
“If it’s not going to help me, it’s still going to advance education and research on Fabry disease,” Primus says. “If this treatment fails after a few years, at least they know this didn’t work and they can try something different.”
Manufacturer Sangamo Therapeutics recently announced preliminary results in the Fabry gene therapy clinical trial. Levels of alpha galactosidase A were sustained in 13 people, and several recipients have been able to go off enzyme replacement therapy. The longest treated participant has been in the study for two years. Long-term data on more people is needed.
In the Fabry gene therapy study, four people have been infused with the viral vector at Emory, and 20 people are now participating across several sites in the United States, the United Kingdom and Australia.
