In the movies, scientists can take a sample of someone’s blood, put it in a mass spectrometer, and quickly determine everything that’s in the sample.
In real life, they can only scratch the surface, because how the body breaks down thousands of chemicals from drugs, foods, cosmetics and other exposures remains uncharted territory or “dark matter”. They know the chemical starting points, but they don’t know all the breakdown products or where those products will show up in their analyses.
Emory researchers have developed a system for generating “xenobiotic metabolites” – the breakdown products – from environmental chemicals, so that they can be analyzed on a large scale. Potentially, the system could be used to establish how chemicals are metabolized and distinguish who has been exposed and how much, even if the original chemical is not present in the body anymore.
“This system allows us to identify the presence of chemicals based on their downstream biotransformation products,” says lead author Ken Liu, PhD, senior scientist in the Clinical Biomarkers Laboratory at Emory University School of Medicine. “Many of the downstream biotransformation products cannot be purchased and otherwise could not be confidently identified.”
The research, published Tuesday in Nature Communications, was a collaboration supported by a grant from the NIH Common Fund’s Metabolomics program and by Emory’s HERCULES Exposome Research Center, both funded by the National Institute of Environmental Health Sciences.
Co-first author of the paper is associate research scientist Choon-Myung Lee, PhD, in the Department of Pharmacology and Chemical Biology. The senior authors are Dean Jones, PhD, professor of medicine and director of the Clinical Biomarkers Laboratory, and Edward Morgan, PhD, professor of pharmacology and chemical biology.
The system uses human liver extracts to metabolize a given chemical, and then analyzes the pattern of the breakdown products using isotopic labeling and high-resolution mass spectrometry. This generates a metabolic signature for researchers to follow to identify the relevant exposure in humans.
“Using this information, we could potentially perform chemical ‘contact tracing’ for an original exposure, even if the original parent chemical is no longer present,” Liu says. “We are trying to capture the diversity of chemical exposures that are present in each individual, since the majority of disease risk is attributed to environmental exposures.”
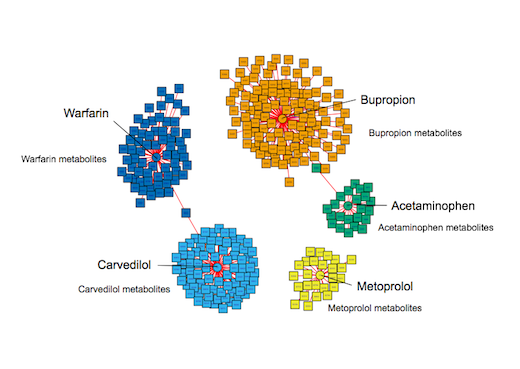
As a demonstration of the system’s capabilities, the researchers used their system to analyze blood samples from Emory University Hospital patients. They could successfully identify those who were taking various medications, such as acetaminophen, the antidepressant bupropion, the blood-thinner warfarin and beta-blockers metoprolol and carvedilol. They were able to confirm exposure based on electronic medical records.
In a second test, the researchers analyzed blood and urine samples from a microbiome clinical trial to look for chemical exposures they didn’t know beforehand.
“We didn't know anything about drug or diet or environmental exposures in these individuals,” Liu says.
The team were able to detect traces of chemicals indicating exposure to nicotine, black pepper, or the heartburn medication omeprazole.
“As additional chemicals and chemical mixtures are processed through this approach, we envision being able to map out the diversity of environmental exposures in the community and identify specific biomarkers that are linked to health outcomes,” Liu says. “Ultimately, adoption of this approach into clinical practice could identify modifiable risk factors for human disease.”
This work was supported by the National Institute of Environmental Health Sciences (U2CES030163, P30ES019776), the National Institutes of Health Director’s Office (S10OD018006), the National Institute of Diabetes and Digestive and Kidney Diseases (RC2DK118619), the National Institute of Allergy and Infectious Diseases (UM1AI104681, K23AI144036), the Georgia Clinical and Translational Science Alliance (UL1TR002378) and a Southern Society for Clinical Investigation Research Scholar Award.