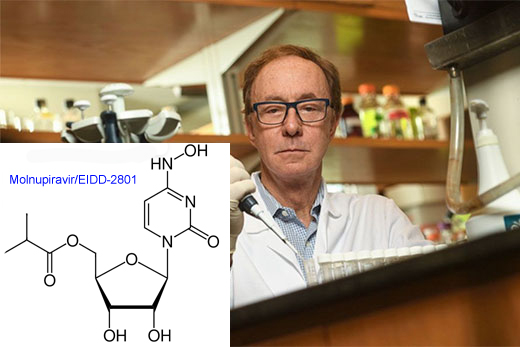
An antiviral drug initially discovered by Emory’s non-profit drug development company DRIVE appears safe and reduces SARS-CoV-2 to undetectable levels in COVID-19 patients after five days of administration, according to data from a Phase II clinical trial in the United States.
Molnupiravir, previously known as EIDD-2801, can be provided as a pill in an outpatient setting, which could be a step up in ease of distribution and convenience. Although remdesivir and antiviral monoclonal antibodies have received Emergency Use Authorizations from the FDA, they must be given intravenously or by injection. In addition, drugs like molnupiravir could flexibly tackle SARS-CoV-2 variants, which have emerged as a concern in recent months.
"There's still an urgent need for an antiviral drug against SARS-CoV-2 that can be easily produced, transported, stored, and administered," says George Painter, PhD, CEO of DRIVE (Drug Innovation Ventures at Emory) and director of the Emory Institute for Drug Development.
When the COVID-19 pandemic began, DRIVE quickly repurposed a broad-spectrum antiviral drug it had been developing against influenza and equine encephalitis. Molnupiravir is being developed further by Merck and its partner Ridgeback Biotherapeutics, a closely held biotechnology company, which licensed the drug from DRIVE last year. All funds for the post-licensing development of EIDD-2801/molnupiravir have been provided by Ridgeback and Merck.
In the most recent clinical study, molnupiravir eliminated infectious coronavirus from nose swabs within five days in all of the people taking it. For comparison, a quarter of people receiving placebo still had detectable virus in their nose swabs at day five.
Emory physicians were not involved in the clinical trial, which recruited 202 adults with COVID-19 symptoms at outpatient clinics in the United States. The data were presented at the recent Conference on Retroviruses and Opportunistic Infections (CROI).
While molnupiravir is proven to interfere with coronavirus replication in infected patients, more data is required to determine whether it can prevent severe illness. Merck and Ridgeback say that more results from the U.S. clinical trial will be shared when they become available, and additional Phase 2 and 2/3 clinical studies are underway. Molnupiravir has also been tested for safety in a clinical trial in the United Kingdom.
Molnupiravir works by forcing the viral enzyme that copies SARS-CoV-2’s genetic material to make so many mistakes that the virus can’t replicate. Still, Merck’s comprehensive testing indicates that high doses of the drug are not mutagenic in animals.
Emory scientists, in collaboration with top coronavirus experts at other universities, have previously shown that EIDD-2801 is highly effective at interfering with coronavirus replication and transmission in animal models and also in mice implanted with human lung tissue. EIDD-2801 has broad spectrum activity against a number of diseases of public health concern, including influenza, SARS-CoV-1, MERS, chikungunya, Ebola and equine encephalitis. The drug was initially developed with the support of the National Institute of Allergy and Infectious Diseases, the Defense Threat Reduction Agency, and the Georgia Research Alliance's Venture Development program.