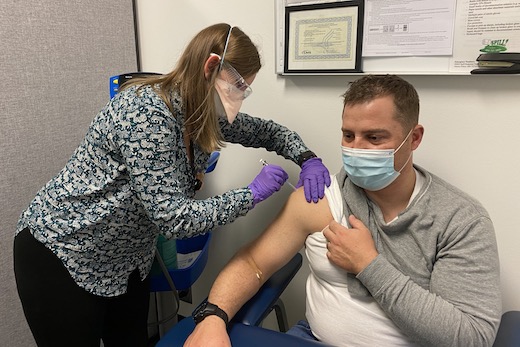
ATLANTA – Emory University is participating in a new Phase 3 vaccine clinical trial for COVID-19. The clinical trial will test the safety and effectiveness of an investigational protein-based vaccine developed by U.S. biotechnology company Novavax, Inc.
Emory administered the first doses of the vaccine to volunteers this week at the Ponce de Leon clinical research site at Grady Memorial Hospital. This is the third late stage vaccine candidate being evaluated by Emory researchers. Colleen Kelley, MD, MPH, associate professor of medicine (infectious diseases) at Emory University School of Medicine, is principal investigator of the study at the Ponce de Leon clinical research site.
“We are thrilled to be launching another COVID-19 vaccine trial after the successes we saw with the Moderna vaccine trial at our research site,” Kelley says. “Multiple, effective vaccines will be necessary to ensure adequate global supplies and conquer the pandemic. This vaccine also has less stringent cold-chain storage requirements than the mRNA vaccines, so that is another plus.”
The Novavax trial aims to enroll up to 30,000 participants in the U.S. and Mexico and expects to include proportional representation among populations most vulnerable to COVID-19 across race, ethnicity, age, and those living with co-morbidities. Participants will randomly receive either the vaccine or placebo in two doses, 21 days apart. Two-thirds of volunteers will receive the vaccine and one-third will receive the placebo.
Novavax’s investigational vaccine does not need to be frozen and can be stored in a refrigerator at 2-8° Celsius, which could facilitate distribution in areas that lack ultracold storage facilities. The vaccine contains no viral material but is made of a protein derived from SARS-CoV-2, which when combined with an adjuvant stimulates the immune response.
Results for a Phase I study on the Novavax vaccine conducted in Australia were published in September by the New England Journal of Medicine, and showed that the vaccine was safe and could stimulate production of antibodies against SARS-CoV-2. At the end of November, Novavax announced that enrollment was complete in a 15,000 participant Phase 3 clinical trial in the United Kingdom and a 4,400 participant Phase 2b efficacy study in South Africa. Results from these studies are expected as soon as the first quarter of 2021.
Emory is among several sites in the United States to test the Novavax vaccine in this Phase 3 trial, supported through the National Institute of Allergy and Infectious Diseases. More information about the study is available at clinicaltrials.gov. The Ponce de Leon clinical research site can be contacted at Atl.ponce.crs@emory.edu.
About the COVID-19 Prevention Network
The COVID-19 Prevention Network (CoVPN) was formed by the National Institute of Allergy and Infectious Diseases (NIAID) at the U.S. National Institutes of Health to respond to the global pandemic. Through the CoVPN, NIAID is leveraging the infectious disease expertise of its existing research networks and global partners to address the pressing need for vaccines and antibodies against SARS-CoV-2. CoVPN will work to develop and conduct studies to ensure rapid and thorough evaluation of vaccines and antibodies for the prevention of COVID-19. The CoVPN is headquartered at the Fred Hutchinson Cancer Research Center. For more information, visit the COVID-19 Prevention Network website.