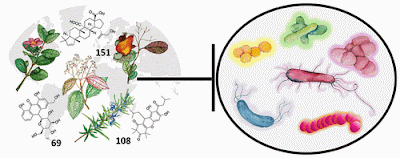
Scientists have compiled the first comprehensive review of plant natural products that play a role in antibacterial activity, to serve as a guide in the search for new drugs to combat antibiotic-resistant pathogens.
Chemical Reviews published the work by researchers at Emory University, which includes 459 plant natural products that met rigorous criteria for demonstrating antibacterial activity. The review is also deposited on the Shared Platform for Antibiotic Research and Knowledge (SPARK), sponsored by Pew Charitable Trusts.
“We hope that chemists and pharmacology researchers will use our review as a guide to dig deeper into the promising potential of many plant compounds,” says Cassandra Quave, senior author of the review and associate professor in Emory’s Center for the Study of Human Health and Emory School of Medicine’s Department of Dermatology. Quave is also a member of the Emory Antibiotic Resistance Center.
In the United States, at least 2.8 million people get antibiotic-resistant infections each year and more than 35,000 people die from them, according to the Centers for Disease Control and Prevention.
“If ever there was a time to cultivate our knowledge and tap into the chemical power of plants, this is it,” Quave says. “We’re seeing a rise in antimicrobial resistance across the globe. And, at the same time, we’re also losing vast amounts of plant biodiversity.”
Two in five plants are currently estimated to be threatened with extinction, according to the State of the World’s Plants and Fungi Report, published in 2020 by the Royal Botanic Gardens, Kew.
Quave is a leader in the field of medical ethnobotany, studying how Indigenous people incorporate plants in healing practices to uncover promising candidates for new drugs. The Quave lab has identified compounds from plants such as the Brazilian peppertree, the American beautyberry and the European chestnut that inhibit dangerous antibiotic-resistant bacteria. Her lab found, for instance, that triterpenoid acids from the Brazilian peppertree “disarm” methicillin-resistant Staphylococcus aureus, known as MRSA, by blocking its ability to produce toxins.
The first antibiotic, penicillin, was derived from microbes in mold that kill bacteria. Since then, scientists have found other microorganisms that live in soil that are easy to grow in a laboratory setting and can kill pathogens resistant to some drugs. The ability of bacteria to continue to evolve resistance, however, is outpacing the ability to generate effective drugs from these sources.
“One obstacle to plant natural products making it into the new drug pipeline is the complexity of the discovery process,” Quave says. “You have to identify a promising plant candidate, tease through the hundreds of chemicals contained within a particular plant to identify the active compound, and then isolate enough of this compound to do experiments on it. It’s not nearly as easy as sequencing a soil microbe and growing up a big vat of it to conduct experiments.”
Tapping the knowledge of traditional people who have used plants for centuries to treat infections offers valuable clues for where to focus research, she adds.
“In recent decades, interest has grown in investigating plants as potential drug candidates,” Quave says. “Technologies have improved to more easily access and study bioactive molecules within plants. And more papers are being published that follow standardized procedures for evaluation of antimicrobial activities among plant compounds.”
For the current review, the Quave lab looked at nearly 200 papers published between 2012 and 2019 that met strict standardization criteria for authenticating plant-derived compounds that significantly inhibited antibacterial activity. The co-authors spanned undergraduates who conducted the initial literature reviews to graduate students and scientists specialized in biology, chemistry, pharmacology and/or botany.
The 459 compounds included in the review encompass a diverse range of species — including those from commonly known plant families such as citrus, daisies, beans and mint. The compounds fall into three major classes of chemicals: About half are phenolic derivatives, around 25 percent are terpenoids, nearly 6 percent are alkaloids and the remainder are classified as other metabolites.
The co-authors selected 183 of the compounds and provided further discussion of their antibacterial activity, biosynthesis, chemical structure, mechanism of action and their potential as antibiotics.
“These are all compounds as they appear in nature, not synthesized or derivatized by chemists,” Quave explains. “We wanted to provide a systematic overview that brings promising drug candidates to the forefront, opening up new chemical space for discovery. Our review can serve as a starting point for chemists to consider whether they could possibly optimize any of these compounds to become scaffolds for antibiotic treatments.”
Co-authors of the review include the following members of the Quave lab: Gina Porras, a post-doctoral fellow specialized in natural products chemistry; François Chassagne, a post-doctoral fellow and a pharmacologist; James Lyles, an associate academic research scientist and analytical chemist; Lewis Marquez, a graduate student of pharmacology; Micah Dettweiler, a former research specialist in the lab who is now a graduate student in agronomy at the University of Florida; Akram Salam, a graduate student of pharmacology; Tharanga Samarakoon, a botanist and collections manager of the Emory University Herbarium; Sarah Shabih, an Emory senior majoring in human health; and Darya Farrokhi, who graduated from Emory in 2020 with a degree in biology.
The work was supported by the National Institute of Allergy and Infectious Disease, the National Center for Complementary and Integrative Health, Emory University and The Jones Center at Ichauway in Georgia.