Christine Dunham's 2018 paper in Proceedings of the National Academy of Sciences, has won that journal’s Cozzarelli Prize.
Biochemist Christine Dunham, PhD and colleagues are being recognized for work on ribosomal frameshifting, a perturbation of the factories that assemble proteins. Their 2018 paper on frameshifting, in Proceedings of the National Academy of Sciences, has won that journal’s Cozzarelli Prize.
The Prize recognizes one outstanding contribution each year to each of the six disciplines represented in the National Academy of Sciences – in the Dunham lab’s case, biological sciences. The Prize, named after the journal’s former editor in chief Nick Cozzarelli, celebrates “scientific excellence and originality.”
Understanding how ribosomal frameshifting occurs helps us grasp not only how proteins are synthesized, but also how some antibiotics disrupt that process and how to re-engineer it for new applications.
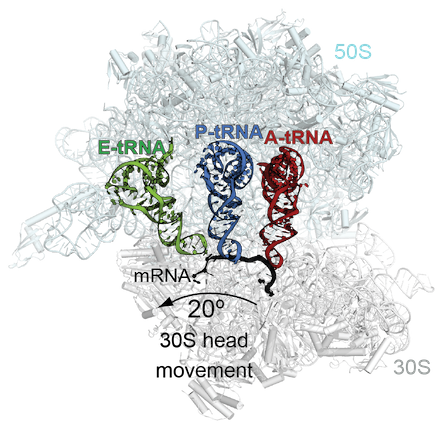
Using X-ray crystallography, the Dunham lab solved the structure of a bacterial ribosome undergoing recoding, influenced by a suppressor tRNA. The title of the paper is: “Mechanism of tRNA-mediated +1 ribosomal frameshifting.” The first authors are postdocs S. Sunita and Samuel Hong, now at MD Anderson.
tRNAs are the adaptors that bring amino acids into the ribosome, and a suppressor tRNA can compensate for a forward frameshift in another gene. The structure shows how the tRNA moves through the ribosome out-of-frame to recode. The tRNA undergoes unusual rearrangements that cause the ribosome to lose its grip on the mRNA frame, and allow the tRNA to form new interactions with the ribosome to shift into a new reading frame.
A commentary in PNAS calls the Dunham lab's paper a “culmination of a half-century quest,” since the mutations in other genes (tRNAs) that suppress frameshifts were identified in the 1960s, yet the molecular mechanism was not substantiated until recent years.