Researchers at Emory University School of Medicine have gained insight into a feature of fragile X syndrome, which is also seen in other neurological and neurodevelopmental disorders.
In a mouse model of fragile X syndrome, homeostatic mechanisms that would normally help brain cells adjust to developmental changes don’t work properly. This helps explain why cortical hyperexcitability, which is linked to sensory sensitivity and seizure susceptibility, gradually appears during brain development.
These physiological insights could help guide clinical research and efforts at early intervention, the scientists say. The results were published Feb. 5 by Cell Reports (open access).
Fragile X syndrome is the most common inherited form of intellectual disability and a leading single-gene cause of autism. Individuals with fragile X syndrome often display sensory sensitivity and some -- about 15 percent-- have seizures.
Scientists’ explanation for these phenomena is cortical hyperexcitability, meaning that the response of the cortex (the outer part of the brain) to sensory input is more than typical. Cortical hyperexcitability has also been observed in the broader category of autism spectrum disorder, as well as migraine or after a stroke.
At Emory, graduate student Pernille Bülow forged a collaboration between Peter Wenner, PhD and Gary Bassell, PhD. Wenner, interested in homeostatic plasticity, and Bassell, an expert in fragile X neurobiology, wanted to investigate why a mechanism called homeostatic intrinsic plasticity does not compensate for the changes in the brain brought about in fragile X syndrome.
Fragile X syndrome is caused by silencing of a single gene, FMR1, preventing production of a critical regulatory protein, FMRP. FMRP both interacts directly with some proteins at synapses, the sites of communication between neurons, and controls the production of others by binding RNA. Neuroscientists are now figuring out how these changes affect neurons and their networks.
Homeostatic intrinsic plasticity or HIP is an important way for the brain to adjust during development, as the brain grows and physiological changes sweep over it. With HIP, brain cells can compensate by modulating their levels of ion channels, which control the transmission of electrical impulses.
“It’s like body temperature,” says Wenner, who is associate professor of physiology. “If someone exercises, the body compensates for the increase in temperature through sweating and changes in blood flow. The analogy here is that the brain is running a bit too hot – but not at baseline. Only if it is pushed.”
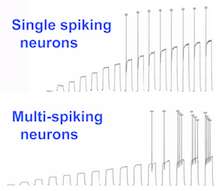
Bülow says she and Wenner were surprised to find that neurons from mice lacking FMR1 did not exhibit altered excitability, compared to normal neurons. This contrasts with the findings of other scientists, and may be because the neurons she studied were from newborn animals, as opposed to animals that were several weeks old, she says.
However, Bülow did find that after a drug-induced calm period, neurons from mice lacking FMR1 had exaggerated responses in their patterns of electrical firing. Chronic treatment with drugs that depress neuronal activity normally evoke homeostatic plasticity as a compensatory effect. In this case, some neurons from the FMR1 knockout mice displayed over-compensation, in terms of the rate of firing multiple action potentials.
This finding led the authors to propose that exaggerated HIP may be a mechanism through which cortical excitability arises during development. They write:
These results are consistent with the observation that symptoms associated with cortical hyperexcitability (seizures, sensory hypersensitivity) do not emerge until a few years into FXS development. These findings support the idea that hyperexcitability is not caused by the changes in ion channel function that are directly produced by the loss of FMRP, but rather indirect compensations to these initial changes.
The researchers are now investigating the biochemical basis for alterations in homeostatic plasticity in fragile X syndrome, and plan on looking at excitability in brain tissues.
“Abnormal homeostatic plasticity could be a convergent mechanism, through which many different neurodevelopmental disorders emerge,” Wenner says.
Co-author T.J. Murphy, PhD, associate professor of pharmacology, contributed to the research.
The research was supported by the National Institute of Mental Health (R01MH109026)and the National Institute of Neurological Disorders and Stroke (R01NS065992).
