To cross the placenta, Zika virus may hijack the route by which acquired immunity is transferred from mother to fetus, new research suggests.
The results were published online Nov. 14 in Cell Host & Microbe.
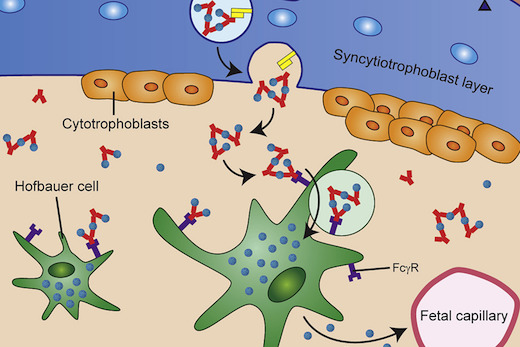
Antibodies against dengue virus make it easier for Zika to infect certain immune cells in the placenta, called Hofbauer cells. This effect was observed in both cell culture and in explanted human placental tissue, says lead author Mehul Suthar, PhD, assistant professor of pediatrics at Emory University School of Medicine and Emory Vaccine Center.
Zika infection during pregnancy can lead to overt microcephaly - a smaller head and brain -- in the developing fetus, as well as more subtle neurological problems detectable later. Researchers had previously observed that syncytiotrophoblasts, cells that make up outermost layer of the placenta, are resistant to Zika infection. Yet studies of Zika-infected pregnant women show that the virus is present in the placenta in the majority of cases.
"We needed to know how the virus gets across the placenta," Suthar says. "Previous studies have shown that Zika persists in the placenta for months. It's clearly getting in there."
However, Suthar says his team's research does not directly address the question of whether having anti-dengue antibodies - as a result of infection or vaccination - will worsen clinical outcomes in Zika infection.
Zika is similar genetically to dengue, they belong to the same flavivirus family and antibodies to one virus sometimes will bind the other. Both are transmitted by the same mosquito vector and their geographical infection patterns overlap in Central and South America, some parts of Africa and South Asia.
There are four strains of dengue virus, and infection with one strain does not lead to long-lasting immunity against the other three. In fact, secondary infection with a different strain can increase the risk of developing a more severe illnesses like dengue shock syndrome and/or dengue hemorrhagic fever. This is thought to happen through "antibody-dependent enhancement": pre-existing antibodies to the first strain, unable to stop the secondary infection, instead bind to immune cells and help the new strain infect them.
On a cellular level, a similar phenomenon is occurring with Zika and dengue. When anti-dengue antibodies are present at the same time as Zika virus, they form complexes (clumps), which are taken up by the placenta. This is a normal process; it's how the mother can transfer acquired immunity to the fetus. The placental cells grab onto antibodies by their Fc receptors, which are unvarying between antibodies. The Zika immune complexes increase viral binding and entry into Hofbauer cells, but also push them to mute antiviral responses.
"This is not the only mechanism," Suthar says. "There may be others.""Once the virus crosses into the placenta, it's down-hill from there," he adds, explaining that the fetal blood-brain barrier is not well-developed and viruses can then access brain tissue directly.
Suthar emphasized that using explanted human placental tissue, it could be possible to look for protective agents that could reduce the probability that Zika will cross the placental barrier. One possibility could be antibodies lacking Fc receptors, he says.
Suthar says his team is planning to investigate similar interactions with other viruses from the same family as dengue and Zika, such as West Nile virus.
Co-first authors are MD/PhD student Matthew Zimmerman and graduate student Kendra Quicke. The laboratories of Jens Wrammert, PhD in Emory's Department of Pediatrics, Rana Chakraborty, MD at the Mayo Clinic and Carolyn Coyne, PhD at University of Pittsburgh Medical Center, contributed to the paper.
The research was supported by National Institute of Allergy and Infectious Diseases (U19AI083019, U01AI131566, 2U19AI057266), the NIH Director's Office of Research Infrastructure Programs (Primate centers: ODP51OD011132), Children's Healthcare of Atlanta and the Georgia Research Alliance.