Know your target. Especially if your target is coming into focus for treating diseases such as schizophrenia and treatment-resistant depression.
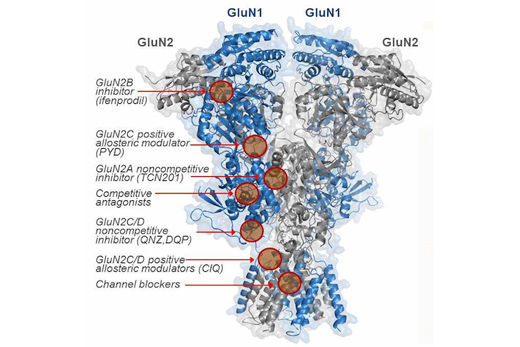
NMDA receptors, critical for learning and memory, are sensors in the brain. Studying them in molecular detail is challenging, because they usually come in four parts, and the parts aren't all the same.
Researchers at Emory have been probing one variety of NMDA receptor assembly found in the cerebellum, and also in the thalamus, a central gateway for sensory inputs, important for cognition, movement and sleep. This variety includes a subunit called GluN2C - together with two partners, GluN1 and GluN2A.
The results were published Thursday, June 28 in Neuron.
Outside of a living brain, NMDA receptor assemblies are typically studied with either two copies of GluN2C or two of GluN2A, but not with one of each, says senior author Stephen Traynelis, PhD, professor of pharmacology at Emory University School of Medicine
"Our data suggest that GluN2C is rarely by itself," Traynelis says. "It's typically paired up with another GluN2 subunit. This means we really don't know what the properties of the main NMDA receptor in the cerebellum or the thalamus are."
Psychiatrists have become interested in GluN2C because it appears to decline in the brains of schizophrenia patients. Mice without adequate levels of GluN2C display abnormalities in learning, memory and sensory processing, which together resemble schizophrenia in humans. In addition, GluN2C appears to be important for the mechanism of ketamine, a drug being studied for its rapid anti-depressant effects.
Using drugs that are selective for particular combinations of NMDA receptor subunits, Traynelis' laboratory showed that an assembly of GluN2A and GluN2C is the dominant mouse form in the cerebellum. When GluN2C is introduced into cortical neurons, it prefers to pair up with GluN2A, the researchers found. This raises the question in regions such as the thalamus of whether GluN2C also appears with a partner GluN2 subunit. They also observed that the GluN2A-GluN2C assembly has distinct electrochemical properties.
Traynelis' laboratory, working with colleagues from Emory's Department of Chemistry, has recently identified compounds that are selective for GluN2C-containing assemblies, and determined which compounds are likely to be active against GluN2C when it is paired up with GluN2A.
"For GluN2C, we believe that the assembly that contains GluN1 and two different GluN2 subunits will be the receptor to study, whether it comes to pharmacology, biophysics, or clinical relevance," he says. Similar efforts are underway to identify the subunit composition of GluN2D-containing receptors, which may also be paired with other subunits.
Postdoctoral fellow Subhrajit Bhattacharya, PhD, and research scientist Alpa Khatri, PhD are co-first authors of the paper. Kasper Hansen, PhD at University of Montana and Sharon Swanger, PhD at Emory also contributed to the paper.
This work was supported by the National Institute for Neurological Disorders and Stroke (NS036654, NS065371, NS097536, NS086361, NS078873) and the National Institute of General Medical Sciences (GM103546).
Traynelis is a member of the Scientific Advisory Board for Sage Therapeutics and a co-founder of NeurOp; both companies are engaged in NMDA receptor drug discovery, although neither was involved in the research in the Neuron paper.