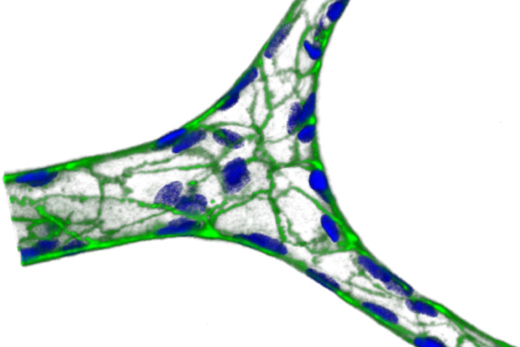
In diseases such as malaria and sickle cell disease, red blood cells break down, with harmful effects on the rest of the body – particularly the lining of small blood vessels.
Biomedical engineers have established a model system for studying these problems, which has potential for use in other cardiovascular diseases as well. The system builds a network of artificial blood vessels, based on familiar “hydrogel” materials that dissolve with heat: gelatin and agarose, a sugar derived from seaweed.
The results were recently published by Nature Biomedical Engineering.
“The key technological innovation here is that our hydrogel can keep these vessels growing for weeks to months, which is much longer than standard approaches,” says senior author Wilbur Lam, MD, PhD. “With this system, we can study not only how vessels respond to aspects of disease but also how well the vessels will heal over time once those insults are removed.”
Lam is assistant professor in the Department of Pediatrics at Emory University School of Medicine and in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University.
Working with Lam, instructor Yongzhi Qiu, PhD and colleagues constructed a hydrogel-based microfluidic device, with branching vessels roughly 20 micrometers across. The device can then be coated by endothelial cells, which line blood vessels, and connected to a pump. An advantage of using hydrogels, the authors write, is that hydrogels are not as stiff as solid polymers, and thus respond in a more physiological relevant manner to changes in fluid flow.
Once fully assembled, the endothelial cells weaken their barriers in response to inflammatory molecules such as TNF-alpha and then heal afterwards.
The researchers also tested responses to heme, a component of hemoglobin formed when red blood cells break open, and they tested the effects of occlusion-prone red blood cells from sickle cell disease patients and red blood cells infected by Plasmodium parasites.
The model system could be used to further dissect the effects of red blood cells vs other cells and inflammatory molecules, and to screen for drugs that could prevent damage to blood vessels, the researchers say.
Co-authors include malaria researcher Tracey Lamb, PhD, formerly at Emory and now at University of Utah, sickle-cell researcher Solomon Ofori-Acquah, PhD, formerly in Emory’s Department of Pediatrics, now at University of Pittsburg, and Clint Joiner, MD, PhD, professor of pediatrics and director of hematology at the Aflac Cancer and Blood Disorders Center and Children’s Healthcare of Atlanta.
The research was supported by the National Heart Lung and Blood Institute (U01HL117721, U54HL112309, R01HL121264) and the National Science Foundation (CAREER Award 1150235, National Nanotechnology Coordinated Infrastructure grant ECCS-1542174).