Most people infected with HIV have neutralizing antibodies: blood proteins produced by the immune system that would eliminate the virus, if it didn’t wriggle away by quickly mutating.
Some infected individuals (20 to 30 percent) develop “broadly neutralizing antibodies,” which can inactivate several varieties of HIV – not just the kind in that person. These antibodies stick to parts of HIV that don’t mutate as much and are common to many viral varieties.
Even in this subgroup of infected patients, broadly neutralizing antibodies develop after a couple years of infection – far too late. Having neutralizing antibodies before viral exposure could possibly protect an individual from infection. A challenge for HIV vaccine design is to figure out how to stimulate the immune system to make broadly neutralizing antibodies more quickly.
A new study by Emory Vaccine Center scientists provides details on how this broadening process occurs. The study tracks how in one Rwandan study participant infected by HIV, the virus escaped an initial attack by the immune system, only to have the immune system come back taking a broader approach. The results were published this week in the journal PLOS Pathogens.
“This is one of the first looks at how antibodies against HIV evolve to come back after a viral escape,” says Cynthia Derdeyn, PhD, associate professor of pathology and laboratory medicine at Emory University School of Medicine, Emory Vaccine Center, and Yerkes National Primate Research Center.
“It starts to answer the question: what does the immune system need to see to develop broadly neutralizing antibodies against HIV? It also provides some information about why the development of breadth takes so long, and occurs in some HIV-infected people but not others.”
The findings have direct implications for HIV vaccine design, and suggest that one way to provoke broadly neutralizing antibodies to form is to have the immune system “chase” a changing virus. Because constantly mutating HIV is a moving target, a successful vaccine may need to educate the immune system in preparation.
Access to patient samples came through collaborations with co-investigators and participants from the International AIDS Vaccine Initiative and the Rwanda Zambia HIV Research Group. The RZHRG program, led by Susan Allen, maintains its home office at the Rollins School of Public Health. This program provides regular follow-up to African couples with one HIV-positive partner that includes HIV-testing, counseling, and condoms. Despite these measures, a low level of HIV transmission still occurs. The collaboration allowed the team to analyze blood samples taken a few weeks after infection occurred and then later as the subject’s immune responses continued.
Working with Derdeyn, graduate student Megan Murphy and her colleagues isolated RNA from the virus that started the infection, and several “escape variants” that appeared as a result of pressure from the immune system. In addition, the team tracked different antibodies produced by the immune system over the course of several months.
Antibodies in the blood are a mixture, produced by immune cells that have variations in their antibody genes. The immune system produces these variations by a process called “somatic hypermutation.” Murphy and her colleagues had to isolate individual variants of immune cells’ antibody genes.
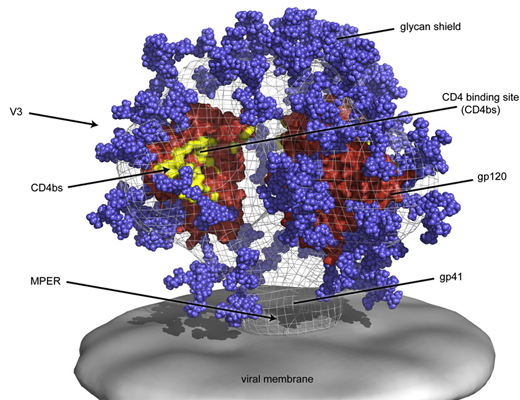
Initially, the Rwandan subject’s immune system produced antibodies that bound to one part of the virus: the base of the envelope’s V3 domain. [See figure above.]
But after two months, the virus had escaped through mutations flanking the V3 domain. Importantly, antibodies that developed after this initial escape neutralized a wider spectrum of the subject’s own viruses but did not yet have activity against viruses isolated from other patients. For a second time, the virus escaped this later wave of antibodies through a different set of changes affecting “glycans,” or sugars attached to viral proteins. These glycan changes in the virus may be especially important for the further development of broadly neutralizing antibodies.
“We think these results are showing us one way that neutralization breadth develops in an HIV-infected individual and how it’s driven by early viral escape," Derdeyn says. "As far as the lessons for vaccine design, we think that the initial antibody target, along with subsequent changes in the virus and their specific order, will be very important to elicit the desired response.”
Murphy is a student in Emory’s Immunology and Molecular Pathogenesis graduate program. Key collaborators included scientists from Emory University, Los Alamos National Laboratory, New York University School of Medicine, and Tulane University. The research was supported by the National Institute of Allergy and Infectious Diseases (AI058706, AI082274, AI088610, AI064060, AI051231), the Emory Center for AIDS Research, and the U.S. Agency for International Development.
M.K. Murphy et al. Viral Escape From Neutralizing Antibodies in Early Subtype A HIV-1 Infection Drives An Increase in Autologous Neutralization Breadth. PLOS Path. (2013).