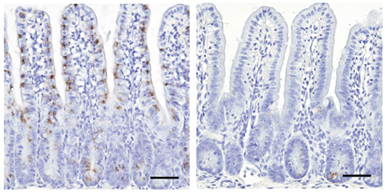
Scientists have found a master regulator gene needed for the development of M cells, a mysterious type of intestinal cell involved in initiating immune responses.
M cells act like “conveyor belts,” ingesting bacteria and transporting substances from the gut into Peyer’s patches, specialized tissues resembling lymph nodes in the intestines. Better knowledge of M cells’ properties could aid research on oral vaccines and inflammatory bowel diseases.
A team of researchers at Emory University School of Medicine and RIKEN Research Center for Allergy and Immunology in Japan has identified the gene Spi-B as responsible for the differentiation of M cells.
The results are published Sunday, June 17 in the journal Nature Immunology.
“This discovery could really unlock a lot of information about the sequence of events needed for M cells to develop and what makes them distinctive,” says co-author Ifor Williams, MD, PhD, associate professor of pathology and laboratory medicine at Emory University School of Medicine. “M cells have been difficult to study because they are relatively rare, they are only found within the Peyer’s patches and can’t be grown in isolation.”
Scientists at RIKEN, led by senior author Hiroshi Ohno, MD, PhD, teamed up with Williams’ laboratory, taking advantage of a discovery by Williams that a protein called RANKL, which is produced by cells in Peyer’s patches, can induce M cell differentiation. Research scientist Takashi Kanaya is first author of the paper.
Kanaya and colleagues found that the gene Spi-B is turned on strongly at early stages of M cell differentiation induced by RANKL. Their suspicion of Spi-B’s critical role was confirmed when they discovered that mice lacking Spi-B do not have functional M cells, and the cells in the intestines lack several other markers usually found on M cells.
“It was somewhat surprising to find Spi-B expressed in intestinal epithelial cells,” Williams says. “Because Spi-B is known to be important for the development of some types of immune cells, it was thought to be expressed only in bone marrow-derived cells.”
In fact, the M cells in Spi-B deficient mice can’t be restored by a transplant of normal bone marrow, the researchers found. That means Spi-B has to be active in intestinal epithelial cells (not immune cells) for M cells to develop.
Williams says information about M cells – in particular, what molecules they have on their surfaces – could be useful for targeting oral vaccines. Most vaccines in use today are administered by injection. But immunologists believe that in some cases, it may be better to deliver vaccines through the mouth or nose, thus strengthening the body's defenses where an infection starts.
Because M cells are involved in the uptake of bacteria, the study of M cells could also guide development of treatments for inflammatory bowel diseases, in which immune responses to intestinal bacteria appear to become dysregulated.
The research at RIKEN was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Japan Society for the Promotion of Science, the Japan Science and Technology Agency, the Japan Science Society, the Takeda Science Foundation, the Mitsubishi Foundation and the Uehara Memorial Foundation.
The research in Williams’ lab is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (5R01DK064730-07) and the Bill & Melinda Gates Foundation.
Reference: T. Kanaya et al. The Ets Transcription Factor Spi-B Is Essential for the Differentiation of Intestinal Microfold (M) Cells. Nat. Immunol. (2012)
doi:10.1038/ni.2352