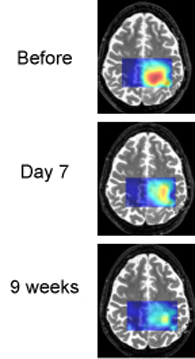
Tumor cells often produce an excess of lactic acid. MRS brain scans show that lactic acid levels are decreasing as treatment proceeds. This patient is an example of a "good responder."
Winship Cancer Institute researchers are testing an experimental therapy for glioblastoma, the most common and most aggressive form of primary brain cancer. The study uses brain imaging in an effort to detect whether the therapy is having an effect after one week.
The therapy combines vorinostat, an experimental drug, with temozolomide, which is standard treatment for glioblastoma.
“Vorinostat is a different type of cancer drug,” says Hyunsuk Shim, PhD, associate professor of radiology at Emory University School of Medicine. “It’s an epigenetic therapy, and the desired effect is to turn genes that could suppress tumor growth back on. One of the desired effects is to restore normal metabolic behavior to the cancer cells, halting tumor growth.”
Epigenetics refers to the study of how genes are packaged or modified, carrying additional information beyond the DNA sequence itself. In many tumor cells, genes that prevent runaway growth in normal cells (tumor suppressor genes) are silenced by epigenetic modification. Inhibiting enzymes called histone deacetylases may reverse this silencing, with possible benefits in treating glioblastoma.
Vorinostat may also help temozolomide, which damages tumor DNA, work better by making tumor cells more sensitive to the drug. Vorinostat, a histone deacetylase inhibitor, is approved by the FDA for CTCL (cutaneous T cell lymphoma) but not brain cancer.
In this National Cancer Institute (NCI)-sponsored clinical trial, the researchers are using magnetic resonance spectroscopy (MRS) to detect changes in brain metabolism brought on by vorinostat. MRS, a form of imaging similar to MRI, allows doctors to monitor the levels of several brain chemicals. The researchers will gauge the levels of inositol and N-acetylaspartate, which are both indicators of healthy brain metabolism.
“This form of therapy may not be effective for all patients, but it is better to figure out as early as possible which patients the drug is working for,” Shim says.
Researchers want to develop new imaging tools to monitor how vorinostat is affecting the tumor. The study is designed to gather information that will allow doctors to make a quick decision on whether vorinostat is effective for a given patient without injecting contrast material.
Shim is collaborating with Jeffrey Olson, MD, professor of neurosurgery, hematology and medical oncology and the co-director of Winship’s brain tumor program, and Xiaoping Hu, PhD, director of Emory’s Biomedical Imaging Technology Center and professor in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University. Hu is a Georgia Research Alliance Eminent Scholar.
For more information about the clinical trial, which currently is enrolling patients, contact 404-778-1900.
The study is being supported by the National Cancer Institute.
Writer: Quinn Eastman
