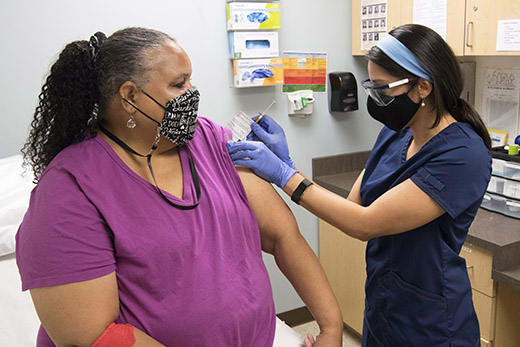
Emory University is taking part in Phase III of a nationwide clinical trial designed to evaluate an investigational vaccine for COVID-19.
Emory administered its first dose of the vaccine at the Hope Clinic of Emory Vaccine Center this week. Hundreds of adult volunteers 18 and older will ultimately be enrolled at three clinics: Emory Children’s Clinic, the Hope Clinic of Emory Vaccine Center, and Grady Health’s Ponce de Leon Center.
“We’re excited to be part of this next critical study in seeking a vaccine to combat COVID-19,” says Evan Anderson, principal investigator for the trial at Emory Children's Clinic, as well as associate professor of medicine and pediatrics at Emory University School of Medicine and Children’s Healthcare of Atlanta.
“As the death toll from this pandemic continues to rise, it becomes even more urgent that we find a safe and effective vaccine to prevent COVID-19. Having this trial take place at Emory gives Atlanta-area residents the opportunity to participate in a study that, if successful, has the potential to help stem the tide of this disease.”
The experimental vaccine, mRNA-1273, was co-developed by researchers at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, and biotech company Moderna, Inc. of Cambridge, Massachusetts.
Emory University was one of three sites that took part in a Phase 1 study of the same vaccine. Early results from that study found the vaccine was generally well tolerated and generated an immune response among participants.
The new larger study (expected to enroll about 30,000 people at more than 80 sites) is designed to test whether the investigational vaccine can effectively prevent COVID-19 infection or prevent severe symptoms and death associated with infection. Participants will be randomly assigned to receive either the tested vaccine or placebo, given in two injections spaced 28 days apart.
People taking part in the study will be monitored for safety through regular clinic visits. Additionally, they will be tested over two years to see who becomes infected in the course of their daily lives. For more information about participating in the study, please see this list of Frequently Asked Questions.
Nadine Rouphael, MD, associate professor of medicine (infectious diseases) at Emory University School of Medicine, is principal investigator for the study at the Hope Clinic, where she is interim director.
Colleen Kelley, MD, MPH, associate professor of medicine (infectious diseases) at Emory University School of Medicine, is principal investigator for the study at the Ponce de Leon Center.
Those interested in participating in the trial at Emory may volunteer through the following:
- Emory Children’s Center (email)
- Ponce Center (email)
The study is supported by Emory’s Vaccine and Treatment Evaluation Unit (VTEU), which is part of NIAID’s Infectious Diseases Clinical Research Consortium (IDCRC) and the COVID-19Prevention Network (CoVPN) supporting this trial. Emory has been a VTEU site since 2007. The consortium is co-led by co-principal investigators David S. Stephens of Emory University School of Medicine, and Kathleen Neuzil of the University of Maryland School of Medicine. Stephens is professor and chair of Emory’s Department of Medicine and vice president for research of the Woodruff Health Sciences Center.
Additional information about the trial design is available at clinicaltrials.gov using the identifier NCT04470427. This trial at Emory was supported in part by the NIAID grant UM1AI148576.