A team of researchers has identified several mutations that cause familial amyotrophic lateral sclerosis (ALS), in a gene that previously had not been linked with the disease.
Emory scientists joined up with investigators at the University of Massachusetts Medical School to make the discovery, reported online Sunday in Nature.
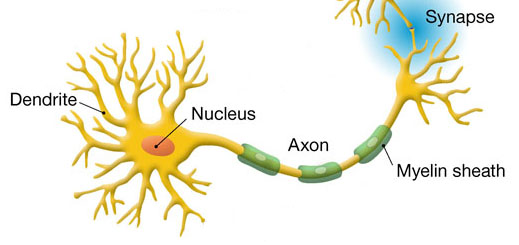
The mutations are in the gene profilin 1 (PFN1), which regulates changes in a cell’s internal skeleton and is essential for the growth of nerve cell axons. The axon is the long projection of a nerve cell that transmits electrical impulses to muscles and other nerve cells.
Mutations in profilin 1 are estimated to account for one to two percent of inherited ALS cases. The finding points to problems in axon growth, triggered by defects in a cell’s internal skeleton, as a potential common mechanism in ALS.
“This discovery identifies what may possibly be a common biological mechanism involved across familial ALS cases,” says John Landers, PhD, associate professor of neurology at UMass Medical School and senior author of the study. “We know of at least three other ALS genes, in addition to PFN1, that adversely impact axon growth. If indeed, this is part of the disease’s mechanism, then it might also be a potential target for therapeutics.”
Led by Landers, the UMass group had been studying families affected by ALS from the United States, Israel and France for several years, with finding new ALS-related genes as one of the goals. They collaborated with Claudia Fallini, PhD, a postdoctoral researcher in Emory University School of Medicine’s Department of Cell Biology, who developed a system for probing PFN1’s function in cultured motor neuron cells.
Wilfried Rossoll, PhD, assistant professor of cell biology, Gary Bassell, PhD, professor of cell biology and neurology, and Jonathan Glass, MD, professor of neurology and director of the Emory ALS Center, are co-authors on the Nature paper.
ALS is a progressive, neurodegenerative disorder affecting the motor neurons in the central nervous system. As motor neurons die, the brain’s ability to send signals to the body’s muscles is compromised. This leads to loss of voluntary muscle movement, paralysis and eventually respiratory failure. The cause of most cases of ALS is not known. Approximately 10 percent of cases are inherited.
The Nature paper describes the discovery of the PFN1 gene mutation among two large ALS families. Both families were negative for known ALS-causing mutations. For each family, two distantly related affected members were selected for exome sequencing.
To identify an ALS-causing mutation, genetic variations between the family members were identified and screened against databases of human genetic variation. This narrowed down the resulting number of potential ALS-causing mutations to two within the first family and three within the second.
Both families contained different mutations within the same gene: PFN1. Additional screening showed that in a total of 274 families affected by ALS, seven had a mutation to the PFN1 gene, establishing it as a likely cause for the disease.
The PFN1 gene encodes a protein that regulates actin, a central component of a cell’s internal skeleton. Landers describes PFN1 as acting like a “railroad tie,” binding together actin’s fibrous filaments and promoting healthy growth of the axon.
When Fallini introduced normal PFN1 into motor neuron cells, it was found diffused throughout the cell. In contrast, the mutant version of PFN1 found in ALS families was found to collect in dense aggregates, keeping it from functioning properly. Motor neurons producing mutated PFN1 displayed markedly shorter axon outgrowth.
“The discovery that mutant PFN1 interferes with axon outgrowth was very exciting to us,” Fallini says. “It suggests that alterations in actin dynamics may be an important mechanism at the basis of motor neuron degeneration.”
Grant support was provided by the National Institute of Neurological Disorders and Stroke (R01 NS065847, R01 NS050557 and RC2-NS070-342) and the Muscular Dystrophy Association (MDA173851).
Reference: C.H. Wu et al. Mutations in the profilin 1 gene cause familial amyotrophic lateral sclerosis. Nature (2012)
doi:10.1038/nature11280