Chatbot opens computational chemistry to nonexperts
New tool turns simulating molecules in solution into a user-friendly chat
Advanced computational software is streamlining quantum chemistry research by automating many of the processes of running molecular simulations. The complicated design of these software packages, however, often limits their use to theoretical chemists trained in specialized computing techniques.
A new web platform developed at Emory University overcomes this limitation with a user-friendly chatbot.
The chatbot guides nonexperts through a multistep process for setting up molecular simulations and visualizing molecules in solution. It enables any chemist — including undergraduate chemistry majors — to configure and execute complex quantum mechanical simulations through chatting.
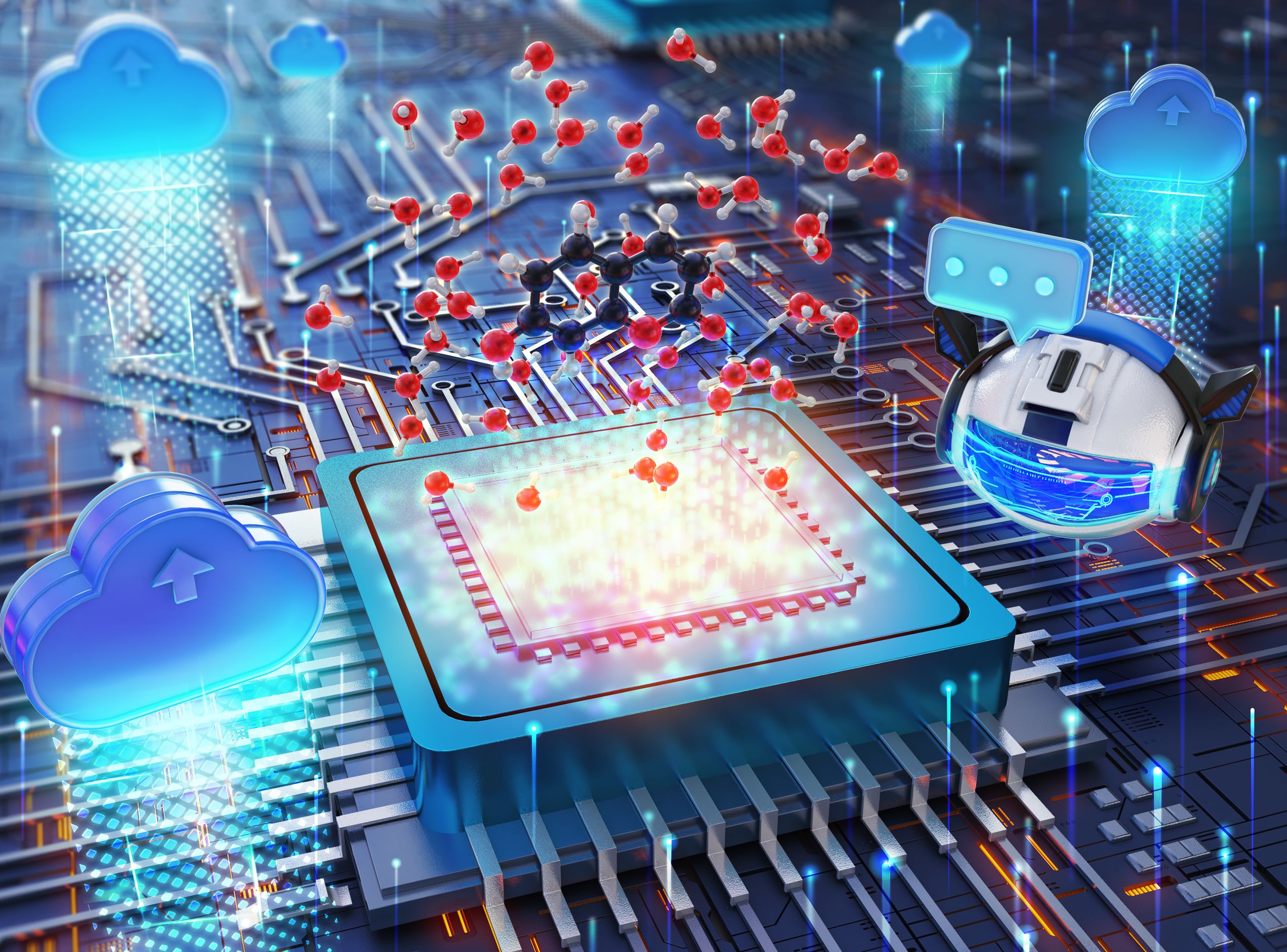
The free, publicly available platform — known as AutoSolvateWeb — operates primarily on cloud infrastructure, further expanding access to sophisticated computational research tools.
The researchers hope that their pioneering work to democratize computational chemistry research will inspire similar initiatives across the natural sciences. (Liu Group/Emory University)
The researchers hope that their pioneering work to democratize computational chemistry research will inspire similar initiatives across the natural sciences. (Liu Group/Emory University)
The journal Chemical Science published a proof-of-concept for AutoSolvateWeb, which marks a significant step forward in the integration of AI into education and scientific research.
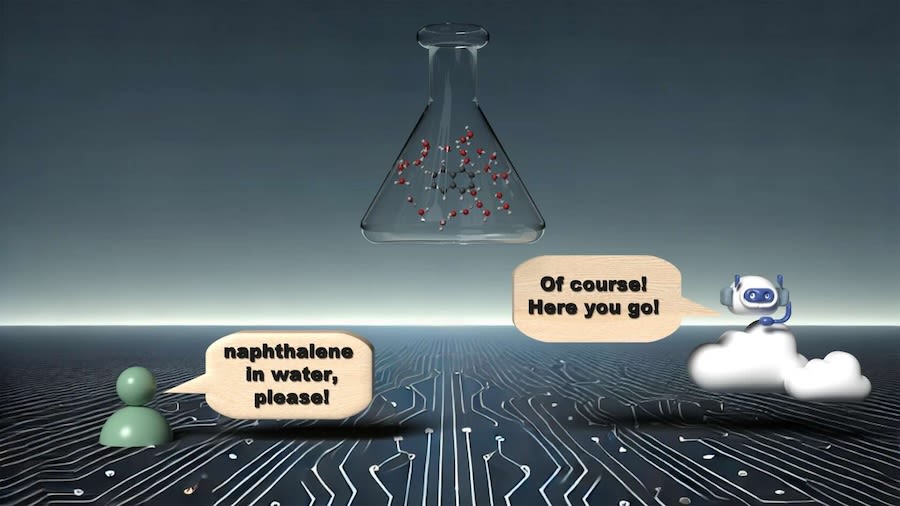
AutoSolvateWeb is geared to set up simulations for a particular chemical to be dissolved (a solute) and a substance to dissolve it in (a solvent), resulting in a solution (a solvate).
The simulations are delivered in the form of 3D movies.
“It’s a bit like a microscope, giving you an atomic-level view of molecules interacting in a solution,” says Fang Liu, Emory assistant professor of chemistry, who led the development of AutoSolvateWeb.
“Chemists can spend less time learning to write computer code so they can focus more of their efforts on specific problems that they want to solve,” says Fang Liu, Emory assistant professor of chemistry. (Photo by Carol Clark/Emory University)
“Chemists can spend less time learning to write computer code so they can focus more of their efforts on specific problems that they want to solve,” says Fang Liu, Emory assistant professor of chemistry. (Photo by Carol Clark/Emory University)
The broad accessibility of AutoSolvateWeb makes it a valuable tool to create large, high-quality datasets addressing the behaviors of molecules in solution. Such datasets provide a foundation to apply machine-learning techniques to drive innovations in everything from renewable energy to human health.
“Our goal is to help speed up scientific discovery,” says Fangning Ren, co-author of the Chemical Science paper and an Emory PhD student of chemistry.
Rohit Gadde, a former Emory research specialist, is first author of the paper. Additional co-authors include Lechen Dong, Emory graduate student of chemistry; Yao Wang, Emory assistant professor of chemistry; Sreelaya Devaguptam, a former Emory visiting scholar; and Rajat Mittal, a former graduate research assistant at Clemson University.
A theoretical chemist, Liu leads a team specializing in computational chemistry, including modeling and deciphering molecular properties and reactions in the solution phase.
Before running a quantum chemistry program for a molecule in solution it’s necessary to determine the geometry of the solute molecule and the location and orientation of the surrounding solvent molecules through molecular simulation. The process of setting up and running these simulations is complicated and time consuming, limiting how often researchers can perform such calculations.
In 2022, the Liu group developed a way to automate many of these calculations with a system it dubbed AutoSolvate. That system reduced the lines of code that a computational chemist needs to enter into a supercomputer to run a simulation from hundreds of lines to just a few lines.
In addition to the command-line interface geared toward more experienced theoretical chemists, AutoSolvate included an intuitive graphical interface suitable for graduate students learning to run simulations.
AutoSolvateWeb builds on this foundation.
The researchers created illustrations to help explain how the chatbot works. (Liu Group/Emory University)
The researchers created illustrations to help explain how the chatbot works. (Liu Group/Emory University)
By operating primarily on cloud infrastructure, AutoSolvateWeb overcomes hardware configuration challenges, further flattening the learning curve for sophisticated computational research. The chatbot communicates via natural language rather than computer code on the front end, while AutoSolvateWeb automates the software processes on the back end.
“Chemists can spend less time learning to write computer code so they can focus more of their efforts on specific problems that they want to solve,” Liu explains. “We also want to enable students to run simulations by themselves so that they can understand the dynamics of molecules in solution more fully.”
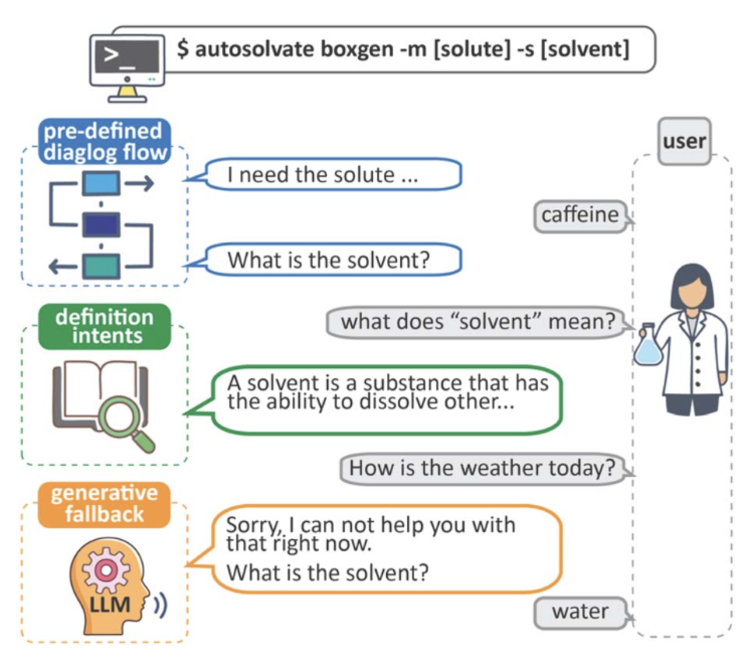
Rather than a large language model (LLM) chatbot, like ChatGPT, the AutoSolvateWeb chatbot is primarily rules-based. It doesn’t converse like a real human over a range of subjects but is geared to specific tasks, similar to chatbots used for customer services like online banking.
Watch a video tutorial of AutoSolvateWeb prepared by the Liu Group.
The chatbot prompts a user to type in the name of a molecule of interest, such as caffeine, then select a solvent to dissolve the caffeine in, such as water. The system taps data from PubChem — the world’s largest collection of freely accessible, online chemical information, assembled by the National Institutes of Health.
The chatbot guides the user step-by-step through the cloud environment, seamlessly integrating multiple open-source software programs needed for the workflow. Once all the proper parameters are calculated through the automated process, AutoSolvateWeb submits the results to a National Science Foundation supercomputer to create the simulation.
The supercomputer returns a trajectory file. The user can download this file and use open-source software to turn the file into a 3D movie of their requested simulation.
AutoSolvateWeb is poised to enhance how chemistry is taught.
“As computers become more and more powerful, they become more important to scientific research,” Ren says. “Undergraduate chemistry students need to get familiar with computer simulations so they can keep pace with advances in how research is done.”
He cites solvatochromism, a technique to analyze the makeup of chemicals in a liquid, as an example of the power of computer simulations for education.
Undergraduate students typically learn about solvatochromism in lab experiments by dissolving a solute known as Riechart’s dye in different solvents. The solution turns blue, red, green or yellow depending on how the solute molecules absorb light.
The simplest explanation for this phenomenon is that the color variations are due to variations in the polarity of a solvent. Changes in polarity stabilize the ground state of a molecule differently, which in turn affects a molecule’s absorption peak along the wavelength of light.
“In science we don't want to just understand what is happening,” says Fangning Ren, PhD student of theoretical chemistry. “We want to know why it is happening.” (Photo by Carol Clark/Emory University)
“In science we don't want to just understand what is happening,” says Fangning Ren, PhD student of theoretical chemistry. “We want to know why it is happening.” (Photo by Carol Clark/Emory University)
What’s trickier to explain are exceptions to this rule. Sometimes solvents of similar polarities produce different colors because of the way hydrogen bonds formed between the solute and the solvent.
“To fully understand how hydrogen bonding plays a special role in this situation, the students need to run a computer simulation,” Liu says. “Seeing is believing. You need to look directly at the structure in motion so that you can understand things at the microscopic scale.”
Such detailed visualizations help students learn to think critically, she says, so they can go beyond memorizing concepts in textbooks to making and analyzing their own discoveries.
Liu and her team is now working to expand the range of chemical systems that AutoSolvateWeb can simulate, going beyond limitations such as single organic molecules as the solute. They are also enhancing the platform’s ability to not only generate data but to store and freely exchange that data across the chemistry community in an open-source format.
The researchers hope that their pioneering work to democratize computational chemistry research will inspire similar initiatives across the natural sciences. Their ultimate goal, Ren explains, is to help connect AI across various domains of basic science, boosting the power of interdisciplinary research.
Story and design by Carol Clark.
To learn more about Emory, please visit:
Emory News Center
Emory University