Plant extract inspires new chemistry and new early lead against triple-negative breast cancer

Plant extract inspires new chemistry and new early lead against triple-negative breast cancer

Chemists at Emory University invented a reaction to streamline the total synthesis of a compound, phaeocaulisin A, extracted from a plant used for centuries in traditional Chinese medicine.
In laboratory dish experiments conducted with biologists at Winship Cancer Institute of Emory University, the researchers showed the compound’s efficacy against HER2-positive breast cancer cells and triple-negative breast cancer cells. An analogue of the compound the chemists constructed boosted this efficacy.
“We not only efficiently replicated a complex natural product,” says Mingji Dai, Emory professor of chemistry. “We also improved upon it by turning it into a more potent compound.”
The Journal of the American Chemical Society published the work, led by Dai and Yong Wan, professor of pharmacology and chemical biology at Emory School of Medicine and director of basic research for the Glenn Family Breast Center at Winship Cancer Institute.
Mingji Dai, right, holds a vial of the synthesized compound alongside collaborator Yong Wan.
Mingji Dai, right, holds a vial of the synthesized compound alongside collaborator Yong Wan.
Phaeocaulisin A is an extract from Curcuma phaeocaulis, a flowering plant in the ginger family native to Asia with various uses in traditional medicine.
“It is only the first step in a long process,” Wan says, “but the new analogue of phaeocaulisin A we have reported shows promising efficacy against triple-negative breast cancer cells, which are very aggressive and challenging to deal with.”
More years of research and testing, Wan explains, first in animal models, are required to further evaluate the compound and determine its potential as a therapeutic treatment.
Meanwhile, the new chemical process offers another tool for constructing complex molecules.
“The icing on the cake,” Dai says, “is that that the chemical reaction we invented holds potential for widespread use in organic chemistry to make many other compounds for drug discovery.”
First author of the study is Chang Liu, who did the work as an Emory PhD student in chemistry in Dai’s lab and has since graduated. Co-authors are Mingyu Zhang, a PhD student in chemistry, and Lidan Zeng, a post-doctoral fellow in Wan’s lab.
"My lab's motto is 'making synthesis beautiful and useful.'"
— Mingji Dai

Dai, Asa Griggs Candler Professor of Chemistry, is also a member of the Discovery and Developmental Therapeutics Research Program at Winship Cancer Institute.
The Dai lab specializes in total synthesis — the construction of complex organic compounds found in nature. His lab has completed the total synthesis of more than 50 natural products with anticancer, antiviral and anti-neurogenerative activities.
Many natural products, or compounds found in plants, soil, deep-sea sponges and fungi, have shown promise for treating various diseases. Penicillin, for instance, was discovered in 1928 when a scientist noticed that mold growing on a petri dish was killing the bacteria in the dish.
Another striking example is Taxol, a landmark cancer drug developed from an extract of the Pacific Yew tree. After decades of research following the discovery of the extract, Taxol went into commercial production in the 1990s.
Examples of plants showing potential for medicinal uses include the Brazilian peppertree, above. In 2017, Emory ethnobotanist Cassandra Quave and colleagues found that an extract from the berries blocks antibiotic resistant bacteria from excreting toxins. (Photo by Ann Watson)
Examples of plants showing potential for medicinal uses include the Brazilian peppertree, above. In 2017, Emory ethnobotanist Cassandra Quave and colleagues found that an extract from the berries blocks antibiotic resistant bacteria from excreting toxins. (Photo by Ann Watson)
Despite the therapeutic potential of some natural products, many challenges are involved in turning them into pharmaceuticals.
“When you isolate a natural product from a plant you often get only a tiny amount,” Dai explains. “One kilogram of a plant may yield only a few milligrams of the natural product, sometimes even less.”
While a few milligrams allow preliminary testing of a natural compound’s efficacy, more material is required to continue research on it. Scientists need to study how to reduce any toxicity a compound shows on human cells while also optimizing its activity against a particular disease.
And if a compound makes it through years of tests and trials, its production must be scaled up for commercial use as a therapeutic.
“My lab’s motto is ‘making synthesis beautiful and useful,’” Dai says. “We use inexpensive, abundant materials to efficiently synthesize these natural products.”
In 2019, for instance, Dai built on work by chemists in China who were looking for molecules with medicinal value in a critically endangered fir tree, Abies beshanzuensis. The Chinese scientists had gathered bark and needles that fell from the last three of these trees still standing in southeastern China.
Dai and his colleagues at Purdue University, where he worked at the time, synthesized two of the molecules extracted from the fir tree and developed analogues of them with minor structural tweaks. Tests showed that one of the analogues was a potent inhibitor of SHP2, an important anti-cancer target in pharmaceutical research.
"We want to bring strategies to neutralize cancer to the clinical bedside."
— Yong Wan

Dai joined the Emory faculty in 2022. Shortly afterward, he met Wan through an event hosted by Emory’s Biological Discovery through Chemical Innovation (BDCI) initiative, a network of investigators working at the intersection of chemistry, biology and human health.
“Emory is a leader nationally in the integration of biology and chemistry,” Wan says. “and the BDCI and the newly established Emory Center for New Medicine are great platforms for bringing people together.”
Wan and Dai bonded quickly. They both come from the same province in China — Sichuan — which is also the home of the giant panda. And both Wan and Dai are passionate about finding treatments for serious diseases.
They began discussing ideas for collaborating, drawing on the complementary strengths of their labs.
“My lab’s focus is to find ways to integrate basic research into translational research,” Wan says. “We are not only trying to understand the mystery of mechanisms behind cancer. We also want to bring strategies to neutralize cancer to the clinical bedside.”

Curcuma phaeocaulis, the source of phaeocausilin A, has been cultivated in Sichuan more than 900 years for traditional medicine. Modern scientific research has identified anti-inflamatory and anticancer activity of phaeocausilin A, including a 2013 paper reporting biological activity of the extract against melanoma cancer cells in lab experiments.
That led Dai and Wan to investigate its potential against various types of breast cancer cells.
Dai compares developing the steps needed to synthesize a natural compound to climbing Mount Everest. The journey is not a success until you reach the peak — the target compound. Researchers typically go down many false trails and impasses along the way.
“Total synthesis is certainly a science, but it is also an art,” Dai says. “The beauty lies in designing a path that leads to the molecule. You see the elegance and ‘scenic beauty’ in the design.”
It’s also one of the best ways to train students, Dai says. “They learn a lot of chemistry, how to plan, manage and execute a project. If anything goes wrong, they may have to reroute by developing a new design.”
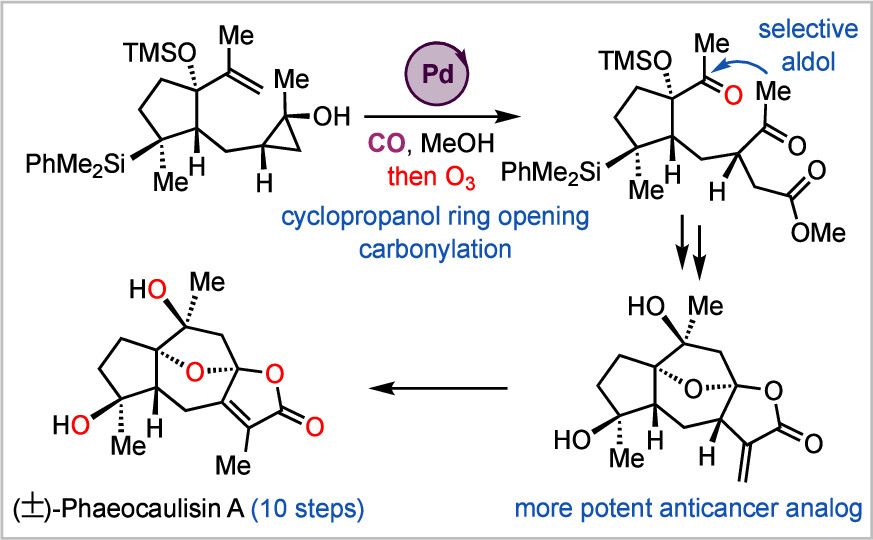
A diagram of the new palladium-catalyzed carbonylation reaction developed by the Dai lab. (ACS Publications)
A diagram of the new palladium-catalyzed carbonylation reaction developed by the Dai lab. (ACS Publications)
Other chemists had previously achieved a total synthesis for phaeocausilin A through a 17-step process. The Emory researchers wanted to find a shorter, more efficient route.
On the way to this goal they invented a new palladium-catalyzed carbonylation reaction, which utilizes cheap and abundant carbon monoxide as a building block. This new reaction helped to streamline their total synthesis of phaeocausilin A to 10 steps.
Going by what Dai describes as “chemist’s intuition,” the researchers hypothesized that the analogue created during step nine of the total synthesis might prove even more potent.
The Wan lab validated this hypothesis, finding that the analogue for phaeocausilin A boosted its activity against HER2 positive breast cancer cells and triple-negative breast cancer cells.
The findings set the stage for further investigation of the compound as a potential breast cancer therapy, a continuing project of the Dai and Wan labs.
“We’re good friends now,” Dai says of his partnership with Wan, “and we have several more ongoing collaborations.”
The work for the current paper was funded by the National Science Foundation.
Story and design by Carol Clark. Photos by Sarah Woods, Emory Photo/Video, except as noted.
To learn more about Emory, please visit:
Emory News Center
Emory University