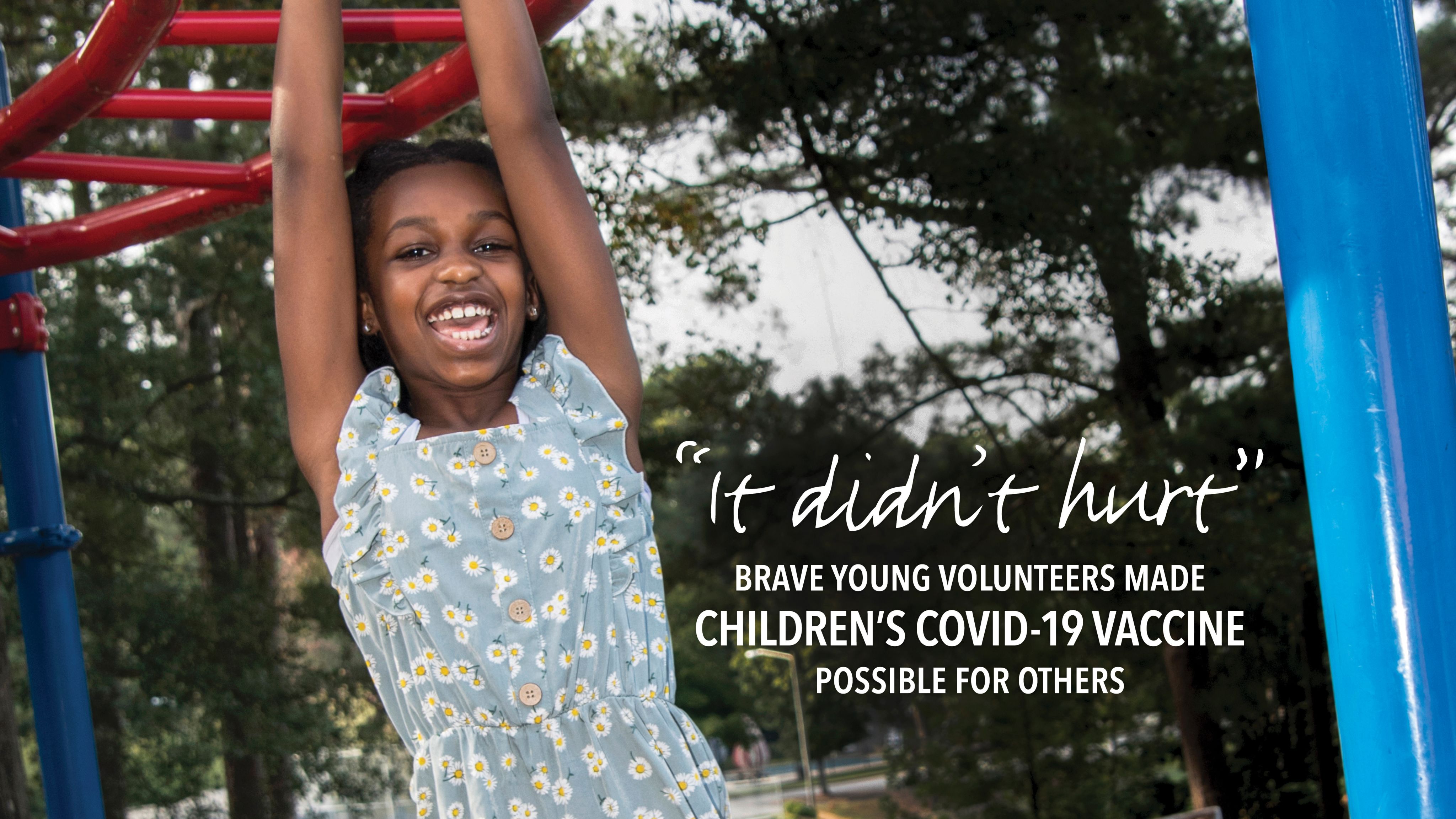Brave young volunteers made children’s COVID-19 vaccine possible for others

THE PLEA “THINK OF THE CHILDREN” HAS BECOME SUSPECT. POLITICIANS DEPLOY THE PHRASE TACTICALLY. OVERWROUGHT CHARACTERS ON THE SIMPSONS USE IT.
Still, early in the COVID-19 pandemic, thinking of the children was precisely what pediatricians and vaccine researchers at Emory were urging everyone to do. They wanted effective vaccines to be available to children as soon as possible—to protect both the children themselves and the adults around them.
Last year, initial studies described few COVID-19 cases among children, and most were mild.
Headlines proclaimed, “Children Mysteriously Untouched by Coronavirus,” “Coronavirus Sparing Kids” And “Why Children Are Faring Better than Adults.” This was before a better understanding of the delayed complications of COVID, the emergence of the more infectious Delta and Omicron variants, and the mid-2021 surge of infections that affected children and adults.

Emory Professor of Medicine and Pediatrics Evan Anderson urgently called for the approval of a well-tested and safe COVID-19 vaccine for children. Photo by Johnathon Kelso
Now one vaccine, from Pfizer and partner BioNTech, has been approved by the Food and Drug Administration (FDA) for children as young as 5. Another, from Moderna, will likely follow. On top of that, booster shots of Pfizer’s vaccine recently became available for teens ages 12 to 15. These are the same vaccines that have been distributed across the country for adults but given at lower doses for children.
The vaccines’ approvals for children are expected to add an important piece to the puzzle of community defense against COVID-19, facilitating a safer return to in-person education, sports, and other activities for children and their families, teachers, and other adults. Early data indicates that despite its increased infectivity, Omicron is not more severe for children, and vaccination is still effective at preventing hospitalization as a result of Omicron infection.
More than 400 young volunteers who participated in clinical trials at the Vaccine Research Clinic at Emory-Children’s Center have been a part of making the vaccine approvals happen. In May 2021, when Emory announced the opening of COVID-19 vaccine studies for children younger than 12, there was strong interest from Atlanta-area parents. Organizers received inquiries from more than 2,000 families, considerably more than the site was allowed to enroll for the 5-11 age group. Both studies are continuing to enroll for younger ages, and participants are being followed closely.
Although the kids who participated got something out of the trials—being vaccinated before their peers—they also took a risk. They were trying something that was then untested in children. They, in turn, provided benefits to others their age as well as adults in the community.

Isabel Saneda, a 10-year-old participant in the trial of the Moderna vaccine
Isabel Saneda, a 10-year-old participant in the trial of the Moderna vaccine
Isabel’s mother, Samantha Saneda, signed her up after a recommendation from a friend. A nurse from the Vaccine Research Clinic called Saneda on a Wednesday with a list of detailed questions. She was told they might need to come in on short notice. The next day, Saneda drove through the pouring rain from Cumming to Atlanta to get her daughter there, canceling a dentist’s appointment along the way. Isabel wasn’t looking forward to a shot, but this one was relatively easy.
“We told Isabel this is helping her friends and people she doesn’t even know,” Saneda says. “At that point, nobody knew about the Delta variant. We feel very fortunate.”

Emory Professor of Medicine and Pediatrics Evan Anderson urgently called for the approval of a well-tested and safe COVID-19 vaccine for children. Photo by Johnathon Kelso
Emory Professor of Medicine and Pediatrics Evan Anderson urgently called for the approval of a well-tested and safe COVID-19 vaccine for children. Photo by Johnathon Kelso

WARP SPEED VERSUS STUCK IN NEUTRAL
The development of COVID-19 vaccines for adults has progressed at “Warp Speed,” as the federal government’s program to accelerate them was called. In the US, three highly effective vaccines have been authorized for adults, and millions of doses have been distributed and delivered.
But last year, vaccines for children were “stuck in neutral,” as Emory Professor of Medicine and Pediatrics Evan Anderson and colleagues from other universities would point out in an influential article that appeared in September 2020 in Clinical Infectious Diseases. Anderson is a pediatrician at Children’s Healthcare of Atlanta and leader of the Vaccine Research Clinic. “We called for initial pediatric studies for COVID-19 vaccines to begin at the same time as the Phase 3 studies for adults,” Anderson says.
Although Pfizer did include teenagers in its 2020 COVID-19 vaccine studies, other vaccine studies in younger children did not begin until months later. Anderson says the delay in testing vaccines for children was partly due to a perception that developed early on that SARS-CoV-2 infection did not affect children as seriously as adults—especially older adults, in whom the mortality risk was highest. The delay was also partly by design. Children are not simply small adults; their physiology and metabolism are different. Children’s immune systems can respond more strongly to vaccines than adults do, and vaccine side effects could be more intense for them. Researchers needed to work out the best and safest dose.

Jack Shaeffer, 11, was one of the volunteers for the children’s COVID-19 vaccine trial.
As it turns out, kids’ apparent invulnerability to COVID-19 was an illusion. Although SARS-CoV-2 infection is often mild or even asymptomatic in children compared with adults, they are clearly capable of being infected. And, when they are, studies show that kids shed about as much virus as infected adults do, so they pose a comparable transmission risk.
“Among the most important methods of COVID-19 transmission are from inside the household and from household visitors,” Anderson says. “Having a vaccine available for our children will have the potential to significantly impact transmission to parents, grandparents, and other adults.”
In addition, though the risk of mortality from COVID-19 is lower for children, they can certainly die from it. As Anderson pointed out last year, more kids had already died from COVID than during the past several flu seasons combined—and kids are routinely given flu shots. For kids, COVID can lead to frightening complications, such as MIS-C (multisystem inflammatory syndrome in children). In the surge of coronavirus infections that began in fall 2021, children were affected more than in previous waves. In Georgia, the proportion of COVID cases among children rose to 30 percent of the total—partly because of the reopening of schools and also because some adults already had been vaccinated.

Jack plays basketball with his older brothers.
THE WORLD GETS BACK TOGETHER
Faced with uncertainty about the risks of COVID, many Georgia school districts closed for in-person teaching and transitioned to online learning. Some parents and children found online learning difficult to manage or access. Schools reopened only to shut down again after local outbreaks.
In Decatur, after much debate, the local school district gave families the option of returning to classrooms in spring 2021. Concerned about the wave of infections at the time, ’s family opted for him to “stay virtual” until the end of the school year. “We didn’t want to go back too early,” says his mother, Suzanne Shaeffer.
But as vaccines became available for adults, Jack began to feel like he was missing out on his favorite activities, such as lacrosse. His father, Francis, a dentist, had participated in the placebo-controlled study of the Moderna vaccine in adults.
Jack missed hanging out with his cousins, while his older brothers were already eligible to be vaccinated. That’s why he was eager to take part in the pediatric study when it started at Emory.
11-year-old Jack Shaeffer
11-year-old Jack Shaeffer
“I was excited,” Jack says. “The faster we do this vaccine, the faster the world gets back together.”
“It’s worth the risk,” he adds. “So many people are dying. It makes me so sad and disappointed.”

Jack is looking forward to returning to his favorite activities, such as lacrosse and band.
Jack’s parents say they all regained some confidence about socializing after Jack was vaccinated as part of the study.
Jack has taken to suggesting names for the alarming viral variants he learns about in the news, favoring mythological characters, such as the Norse god Loki.
“He floats a new name for the variants each time we go to the clinic,” his mother says, laughing.

Jack Shaeffer, 11, was one of the volunteers for the children’s COVID-19 vaccine trial.
Jack Shaeffer, 11, was one of the volunteers for the children’s COVID-19 vaccine trial.

Jack plays basketball with his older brothers.
Jack plays basketball with his older brothers.

Jack is looking forward to returning to his favorite activities, such as lacrosse and band.
Jack is looking forward to returning to his favorite activities, such as lacrosse and band.

THE POSSIBILITY OF A SALINE SHOT
The studies at Emory’s Vaccine Research Clinic were testing the same mRNA vaccines that were approved in adults, but they came in two stages. The first tested various doses. Participants and their families knew they were getting a known amount of the vaccine. The uncertainty that occurs in some clinical trials, about whether participants received a placebo or the actual drug, was not present.
The second stage of the vaccine studies was placebo-controlled, using information gleaned from the first stage to set the doses. For the Moderna study, investigators decided on a dose for children ages 6-11 that was half the adult dose. This produced a level of coronavirus-neutralizing antibodies that was actually greater than the response seen in young adults immunized with the higher dose.

9-year-old Autumn Gilford
9-year-old Autumn Gilford

Autumn Gilford, 9, is a participant in the second stage of the Moderna pediatric vaccine study.
Although more study participants received active vaccine than placebo (the odds were 3:1), young volunteers were still made aware of the uncertainty. “I didn’t know if it was going to be a COVID shot or a saline shot,” says 9-year-old Autumn Gilford, a participant in the second stage of the Moderna pediatric vaccine study.
Autumn says she wasn’t scared of the shots. Her younger brother, London, is signed up to participate in the next stage of the pediatric studies, for children under five. “I thought it was beneficial,” says their mother, Rashante Harris. “We just wanted to do something.”

Autumn and her younger brother, London, play in a park near their home.
“If anything, I’m the one posing a risk to Autumn,” she adds, because of her job as a physician at a local urgent-care clinic. Harris was among the first to be vaccinated in December 2020.
Autumn and her family have been cautious about venturing back out even after businesses in Georgia were reopened last year. They’ve avoided restaurants and parties. “She’s more cautious than I am,” says Harris’s husband, Michael Gilford, whose job at Georgia Power is performed out of the office. “At our clinic, we have been seeing people getting sicker,” Harris says.
Harris acknowledged the history of distrust among Black communities for medical research conducted by white-dominated institutions. As a physician, Harris’s familiarity with vaccines and research made crossing that bridge easier. “I didn’t tell most people [about Autumn’s participation in the study],” Harris says. “People who know me would come to me if they had questions.”

Autumn plays in a park near her home.

Autumn Gilford, 9, is a participant in the second stage of the Moderna pediatric vaccine study.
Autumn Gilford, 9, is a participant in the second stage of the Moderna pediatric vaccine study.

Autumn and her younger brother, London, play in a park near their home.
Autumn and her younger brother, London, play in a park near their home.

Autumn plays in a park near her home.
Autumn plays in a park near her home.
CHECK OF SAFETY
One purpose of the pediatric vaccine studies is to look for harmful side effects; the health of the young participants was closely monitored. The pediatric studies were not expected to provide statistical confirmation that the vaccines prevented COVID-19 in the same way as adult studies performed in 2020 did. Instead, researchers monitored kids’ antibody levels and other immune responses to see whether the vaccines were likely to be effective, an approach called “immunobridging.”
In summer 2020, reports emerged of teenagers—predominantly males—experiencing myocarditis or pericarditis (inflammation of the heart muscle or lining) after immunization with the mRNA vaccines. Symptoms such as chest pain brought them to the hospital, where doctors could detect signs of heart trouble, such as elevated levels of the cardiac marker troponin or altered electrocardiogram or cardiac MRI scans. For most young patients, the symptoms resolved with conservative treatment after a few days and they were able to return home.
Myocarditis or pericarditis cases in adults and teenagers appear to be rare enough that the 2020 vaccine studies, with tens of thousands of participants, did not catch them beforehand. This summer, the FDA asked Moderna and Pfizer to increase the size of their pediatric studies so more data on these events might be available. Preliminary results from the Pfizer pediatric study indicate no cases of myocarditis reported in children under age 12.
According to data from the Centers for Disease Control and Prevention (CDC), the risk of myocarditis or pericarditis occurring after Pfizer/BioNTech vaccination in teens is about 37 cases for every million doses. The risk of developing myocarditis from coronavirus infection itself is much higher. CDC calculations say that in boys ages 16-17, vaccination with the Pfizer/BioNTech vaccine could be expected to prevent more than 50,000 cases of COVID-19 and 500 ensuing hospitalizations for every million doses distributed. That would have to be balanced against about 70 cases of myocarditis. A similar risk-benefit calculation could be worked out for older age groups; the risk for myocarditis after vaccination appears to decrease after puberty. “Carefully conducted studies have been able to pinpoint the risks associated with these vaccines,” Anderson says. “The benefit to teenagers of vaccination clearly outweighs the miniscule risk.”
LOOKING AHEAD
Anderson recalls that last spring, because of the crush of the pandemic and the urgency of starting vaccine studies for adults, “there was barely any time to think. Everything had gotten very distorted by the pandemic and I often felt like I was in the middle of one of Picasso’s paintings,” he says.
He credits his colleague, pediatrician Carol Kao, with pushing him to complete an article with her for the journal Clinical Infectious Diseases, on “The Importance of Advancing SARS-CoV-2 Vaccines in Children.”
The article had a snowball effect and helped prompt the National Institute of Allergy and Infectious Diseases (NIAID) to develop template plans for pediatric vaccine studies. Anderson’s Vaccine Research Clinic is part of Emory’s Vaccine Treatment and Evaluation Unit, one of several in a network supported by NIAID. That effort also led to the “stuck in neutral” paper, media attention, and pressure from organizations such as the American Academy of Pediatrics.
Seeing the delay in pediatric COVID-19 vaccine trials, some experts have been arguing that COVID-19 exposed a mismatch between the normal processes of vaccine development and the challenge the pandemic posed to society. Back in 2003, Congress passed the Pediatric Research Equity Act, giving the FDA the authority to require pediatric studies for drugs and products such as vaccines. Looking ahead to future infectious disease threats, a proposed strengthening of the act’s policies could require concurrent enrollment of children in clinical trials once initial safety studies in adults have been completed.
After vaccines become available for children, the “real world” test is whether parents accept them. Surveys indicate that parents are roughly divided between those eager to have their children vaccinated and those who are skeptical, with parents in between taking a “wait and see” attitude. Parents are often more risk-adverse on behalf of their kids than for themselves.
Indeed, researchers at Children’s Healthcare of Atlanta asked the parents of children who had been diagnosed with COVID-19 last year about their plans. Only about half said they intended to have their children vaccinated when a vaccine became available.
Common reasons for declining vaccination were concerns about side effects or safety. The rate of vaccine acceptance, however, increased to almost 70 percent when the survey included information about the objective effectiveness of the vaccine in adults.
“We’ve seen the dramatic effects of COVID-19 upon children’s health, mental outlook, and well-being,” Anderson says. “The approval of a well-tested and safe vaccine for children will help parents get their children safely back to school and something resembling prepandemic life.”
Written by Quinn Eastman, photos by Jack Kearse, design by Peta Westmaas
Severe COVID-related Illness in Children
Multisystem inflammatory syndrome in children (MIS-C) is a rare but serious complication of coronavirus infection. It appears to be a delayed response, emerging weeks after an initial COVID-19 infection that is often asymptomatic.
A paper published in July by researchers from Emory and Children’s Healthcare of Atlanta describes five patients who developed neuropsychiatric symptoms as a consequence of COVID-19 or MIS-C. These symptoms may result from viral infection of the brain, as well as the immune system’s inflammatory response to the infection.
Three of the five patients developed acute psychotic symptoms, such as hallucinations. One, an 11-year-old girl, was a recent immigrant to the United States, who was temporarily separated from her parents. She developed respiratory failure and went into shock, then experienced hallucinations and agitation while in the hospital. Her altered mental state lasted a few days, dissipating after treatment with anti-inflammatory drugs. (The children with MIS-C were treated with anti-inflammatory corticosteroids and another immune-calming drug, intravenous immunoglobulin.)
Another of the three was a 15-year-old male who, after becoming ill, displayed “obsession with numbers, hyper-religiosity, hyper-sexuality, visual hallucinations, fast speech, and disorganized thoughts,” according to the paper.
Two of the five were younger—less than two years old—and they experienced seizures and altered mental status. Doctors know that all of the children were infected with the coronavirus because their blood or spinal fluid contained antibodies against it, but sometimes their nasal swabs tested negative for the genetic material of the virus itself.
“There has been an increase in psychiatric symptoms in general during the pandemic, due to factors outside of COVID and inflammation,” says the lead author, pediatric neurologist Grace Gombolay. “These factors include anxiety about the pandemic and COVID itself, social isolation, economic stressors, loss of loved ones, and how our lives have changed. Even though these patients received immune therapy related to MIS-C, most patients with psychiatric symptoms improve with medication and do not need immunotherapy.”
Even though neuropsychiatric symptoms from pediatric COVID-19 or MIS-C are frightening, they’re relatively rare, says Emory Assistant Professor of Pediatrics Christy Rostad, an infectious disease specialist at Children’s Healthcare of Atlanta. What brings kids to the hospital are persistent fever, abdominal pain, vomiting, diarrhea, and rashes. Children with MIS-C can develop low blood pressure (shock) or cardiac problems, such as chest pain and an irregular heartbeat, requiring intensive care.“Those are what we worry about the most,” Rostad says.