GAINING GROUND ON LUNG CANCER
Charting a new future for patients like Brandi
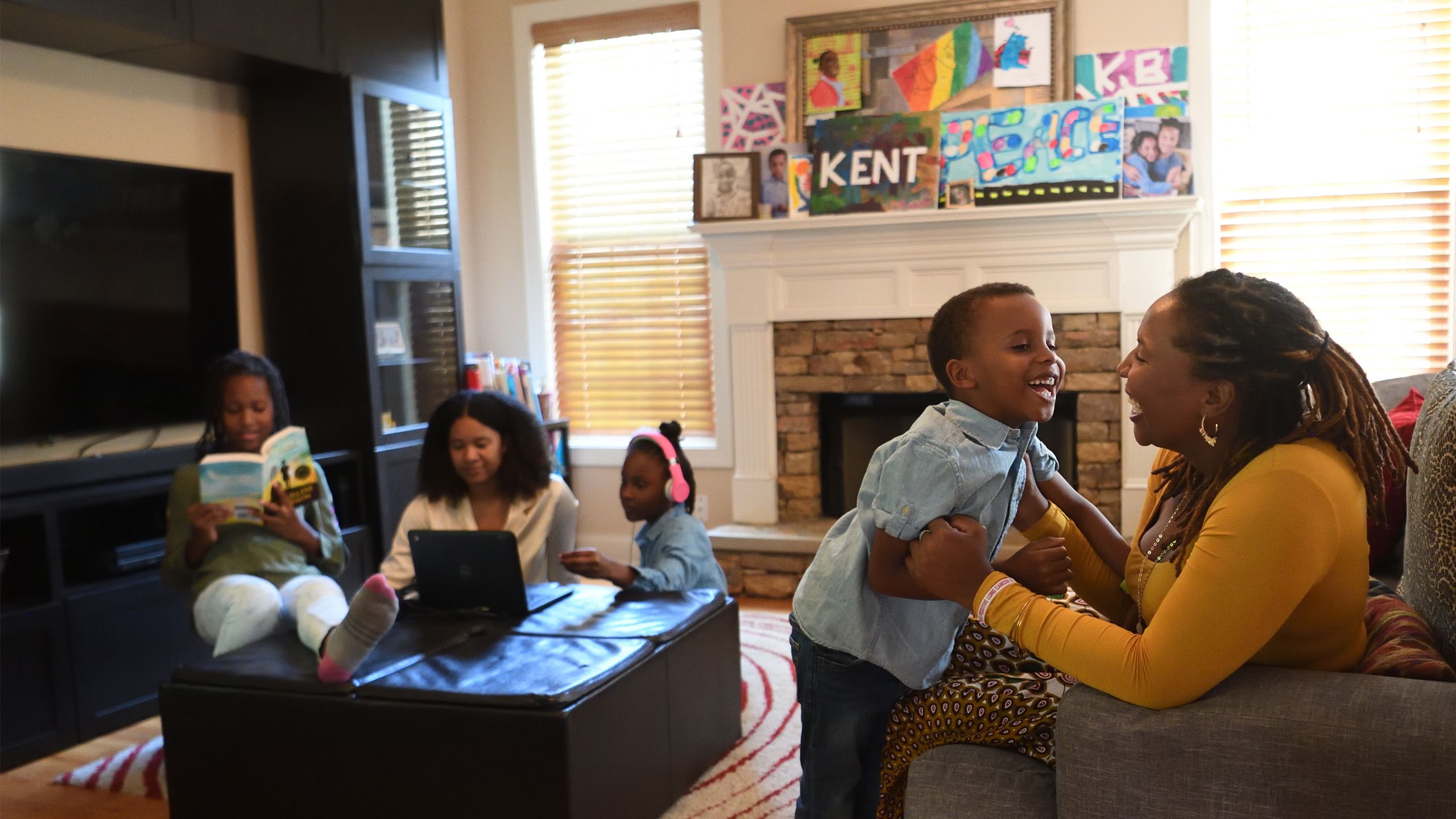
IT STARTED WITH A COUGH.
Just a small cough, nothing painful and certainly nothing Brandi Bryant had any time to worry about.
A mother of four young children with a career as a publication coordinator, Bryant led a full, active life. In the little time she had to herself, she kept fit, sometimes walking as much as 30 miles a week. She had never smoked.
But the cough turned into shortness of breath. Soon after, Bryant, then 39 years old, received a diagnosis of stage III non-small cell lung cancer (NSCLC), the most common type of lung cancer.
“I break down in the office,” she wrote on her blog, recalling her thoughts in that life-changing moment.
“I am the one who takes care of EVERYTHING in my house…
I CANNOT be sick.”
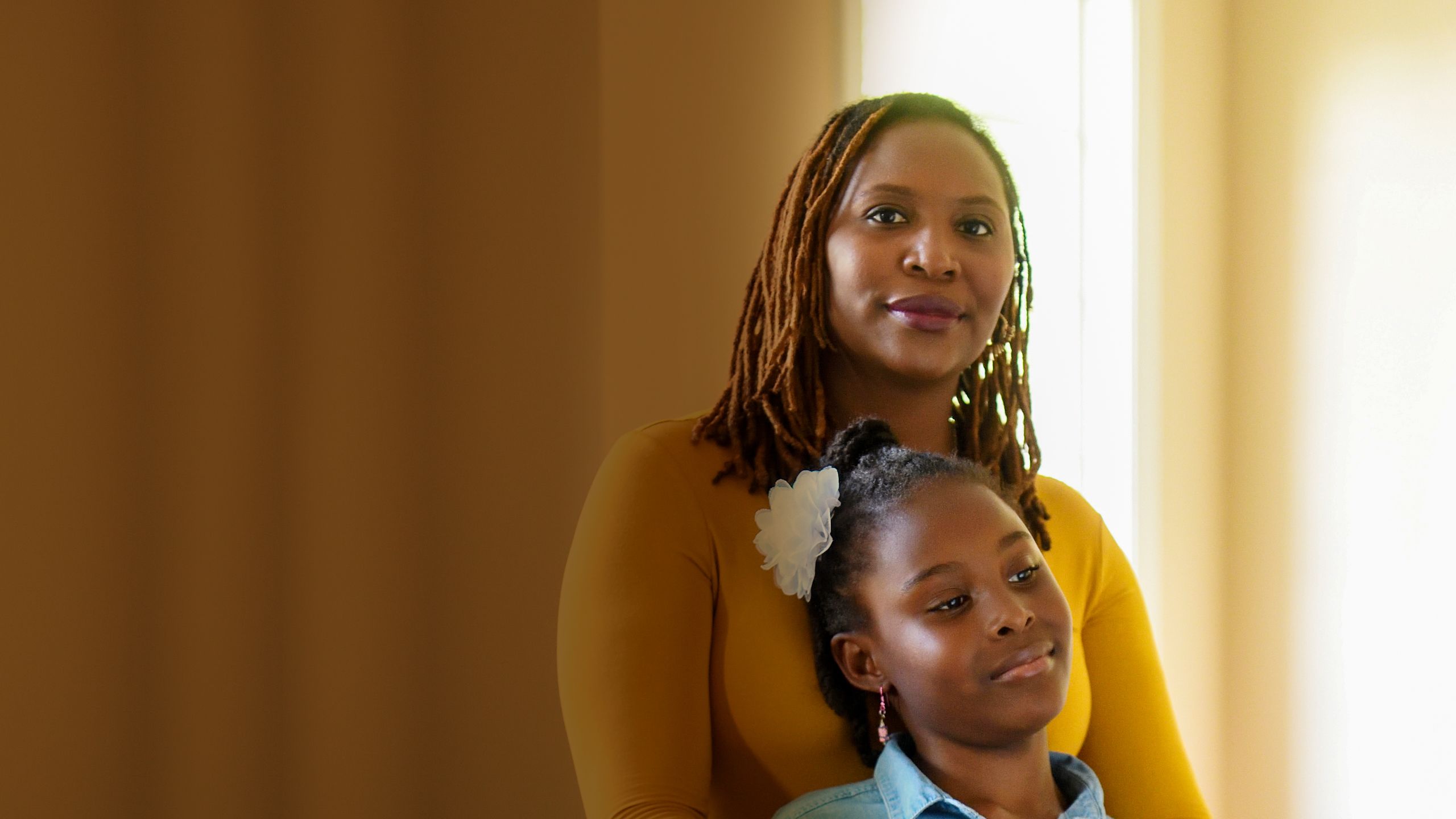
LUNG CANCER HAS LONG BEEN DIFFICULT TO TREAT. Most cases are diagnosed at an advanced stage, and a decade ago, few options were available besides radiation and chemotherapy, with modest effectiveness. That is starting to change. Recent years have seen the advent of immunotherapy, in which the patient’s immune system is re-activated to attack the disease, and targeted therapies, chosen according to the genetic makeup of a patient’s tumors.

These treatments are capable of beating back even advanced cancers, making them appear to vanish from the body. Yet they have their limitations. Immunotherapy drugs are only effective in about 20 percent of patients with lung cancer and can have severe side effects. Similarly, targeted therapies are relevant for just a fraction of lung cancers, and often stop working when the tumor cells they target become drug resistant.
At Winship Cancer Institute, lung cancer specialists think their field is at the beginning of a transformation. They want to make the benefits of the new treatments available to a larger number of patients.

Lung Cancer SPORE team members
Lung Cancer SPORE team members
“In the next few years, we plan to bring innovative approaches to improve the effectiveness of existing therapies for each lung cancer patient,” says Winship Deputy Director Suresh Ramalingam, co-leader of Winship's Lung Cancer SPORE grant. “We want to develop personalized immunotherapy and targeted therapy options.”

With those goals in mind, the National Cancer Institute recently awarded Winship a five-year, $9.7 million Lung Cancer Specialized Program of Research Excellence (SPORE) grant, one of just four in the country. Winship’s Lung Cancer SPORE program includes three research projects, each aimed at strengthening a major tactic against lung cancer. It incorporates clinical trials examining immunotherapy and targeted therapy, and more basic research aimed at sensitizing cancer cells to radiation.
“This grant shows how we weave together basic science, translational, and clinical research, and it will spark new ways of thinking about the problems our patients face,” says Winship Executive Director Wally Curran.
The grant brings together experts not just in lung cancer, but in immunology, pharmacology, drug development, bioethics, and other areas. In addition, it is designed to nurture younger researchers, and allow them to test ideas that they haven’t had a chance to implement, says Haian Fu, leader of Winship’s Discovery & Developmental Therapeutics Research Program and Ramalingam’s counterpart as co-leader of this SPORE grant.
“We bring new people into the field of lung cancer, and we also bring the cutting-edge research in other fields to bear on lung cancer,” Fu says.





When she began her treatment journey in January 2018, Brandi Bryant and her medical oncologist Rathi Pillai and radiation oncologist Kristin Higgins, had agreed on a plan: six rounds of chemotherapy and 30 rounds of radiation followed by 12 months on immunotherapy. This was standard and was considered a curative approach, Pillai says.

As she neared the end of radiation, though, Bryant was so weak she couldn’t climb stairs, couldn’t brush her toddler’s teeth, couldn’t even sit with her kids for more than a few moments. To make matters worse, what had been a small pericardial effusion, a sack of fluid surrounding her heart, had grown so large that it now required an operation. Surgeons were able to drain the fluid, but when they tested it, they found more cancer.
Days later, her cancer was reclassified to recurrent. Ironically, this opened a door for Bryant.
A biopsy found that her tumors had a genetic rearrangement in a gene called ALK (anaplastic lymphoma kinase), which is only present in about 4 percent of lung cancers.
Winship’s Lung Cancer Tumor Board discussed the different options: stick with the original treatment plan, which included immunotherapy, or start her instead on a targeted therapy aimed at ALK. Bryant knew that immunotherapy had produced stunning improvements in some patients, but for many others it did nothing at all. Pillai came down on the side of targeted therapy, recommending a drug called alectinib.
Radiation had irritated Bryant’s esophagus and left her weak and unable to manage solid foods. She didn’t start her first pills until May. But soon, she could sense a change. “I knew something was working,” Bryant says. “I could tell the tumor was shrinking.” As the weeks went by, she began to feel better and better—less fatigued by the daily chores, more like the healthy person she’d always been. She began to walk up stairs again, and took a trip to see family in Oklahoma with all four kids. “Look at me getting my Independent Woman badge back,” she wrote on her blog.


Then, on October 1, exactly five months after she’d started on alectinib, a scan came back with stunning news: no evidence of disease. Bryant was ecstatic. “Hooray!!” she wrote on her blog. “No active cancer, everything looks good!”
For Bryant, targeted therapy has done wonders. Unlike chemotherapy, which kills cancer cells and normal cells, the targeted therapy blocks growth signals that are specific to her cancer cells. Targeted therapies have become a cornerstone of precision medicine. But there’s a catch: they only work for so long. Eventually, a patient’s tumors can become resistant to the drugs, rendering them ineffective.
The hope for patients like Bryant is that by the time that window closes, there will be something else. A year later, she continues to take alectinib pills.
“Four in the morning, four at night,” she says with a chuckle, reciting her daily regimen. “And always with food!”
In the fall of 2017, Ramalingam and colleagues reported the results of a landmark study of the drug osimertinib, a targeted therapy developed for the treatment of non-small cell lung cancers with mutations in the epidermal growth factor receptor (EGFR) gene. In lung cancers, mutations in the EGFR gene are more common than in ALK (15 percent compared to 4 percent).
What makes osimertinib exciting is that it blocks not only the EGFR pathway but also another pathway cancer cells use to escape and develop resistance—a kind of backstreet the tumor takes when the freeway is closed. “With osimertinib, you could block both of those roads at the same time, and that results in better outcomes,” Ramalingam says.
Osimertinib stops tumors from progressing for almost twice as long as previous therapies. Still, cancer seems always to find another way.

Research ethicist Rebecca Pentz and team are conducting a study to determine how best to inform patients about “window of opportunity” trials that add to research knowledge.
Research ethicist Rebecca Pentz and team are conducting a study to determine how best to inform patients about “window of opportunity” trials that add to research knowledge.
“Once osimertinib fails, the patient has to go on chemotherapy,” says Taofeek Owonikoko, a medical oncologist specializing in lung cancer.
With support from the Lung Cancer SPORE, Owonikoko is collaborating with Douglas Graham, chief of the Aflac Cancer and Blood Disorders Center of Children’s Healthcare of Atlanta, to probe a third pathway, called MER, which comes into play when EGFR is blocked. Early findings indicate that MER is active in the tumor microenvironment, where it suppresses the body’s innate immune response. That suggests that blocking the MER pathway might sensitize EGFR-mutated tumors to osimertinib, enhancing that drug’s effect.
Graham and Owonikoko are conducting a clinical trial that combines osimertinib with a new targeted therapy, which was designed to block the MER pathway. That drug, developed in Graham’s lab, is already being studied at Winship in clinical trials. The SPORE will build on this by developing a trial combining osimertinib with the MER inhibitor. If successful, the combination trial could lead to a more effective approach to overcoming resistance to osimertinib.
“We’re really excited about this work, which has direct implications for our patients, both in terms of quality of life and longevity,” says Owonikoko.

The Deng lab is focused on one of the most common mutations driving lung cancer, in the K-ras gene. Here, Dongkyoo Park prepares lung cancer cells to be radiated.
The Deng lab is focused on one of the most common mutations driving lung cancer, in the K-ras gene. Here, Dongkyoo Park prepares lung cancer cells to be radiated.
The Lung Cancer SPORE grant is also supporting basic scientists as they go back to the lab and look for other ways to target mutations in lung cancers. Xingming Deng, a leader in developmental therapeutics, and his colleagues are focused on mutations in the gene K-ras, one of the most common mutations driving lung cancers (more than 25 percent). They have developed a compound that could sensitize cancer cells to radiation by targeting a molecule activated by K-ras, thought to be “undruggable” until recently.
The need for more answers and more options is urgent. Winship physicians treat many lung cancer patients who don’t benefit from the new therapies. Michael Winship is one of them.
Michael Winship was at the beach for a long Labor Day weekend when a friend noticed a lump on his shoulder. It had been years since Winship, 57, had seen a doctor, but he happened to have an upcoming appointment about an issue with his blood pressure.
“I showed the lump to the doctor, and she immediately ordered a CT scan,” he says. The next day he learned he has small cell lung cancer.
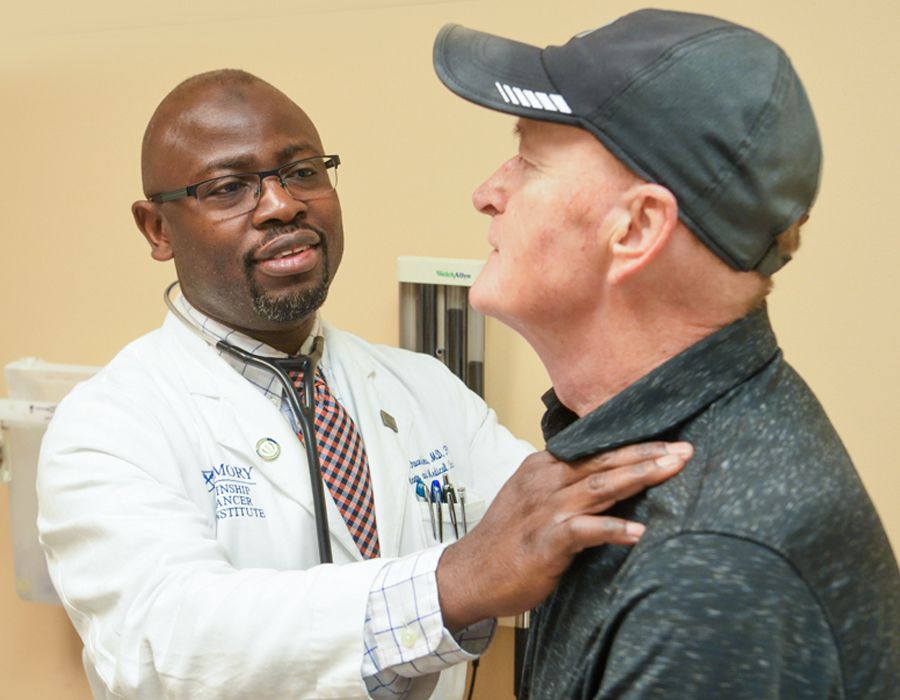
Taofeek Owonikoko examines lung cancer patient Michael Winship.
Taofeek Owonikoko examines lung cancer patient Michael Winship.
Winship was referred to Owonikoko, who promptly started him on radiation and chemotherapy—and that worked well. But once Winship completed the regimen, the tumor on his shoulder began to grow back. “That’s when they switched me to immunotherapy.”
Immunotherapy did not work for him and Owonikoko returned to the drawing board. In the months since, Winship has started another chemotherapy regimen.
Patients like Michael Winship show the limitations of the current therapies and spur the work of Winship’s Lung Cancer SPORE.
Ahmed says several factors may explain why immunotherapy drugs aren’t effective for most lung cancer patients.

“The first priority is understanding how it’s working, why it’s working, and then see when it’s not working, what’s missing. Otherwise you’re in the dark.”
Rafi Ahmed
Director Emory Vaccine Center
Lung Cancer SPORE investigator

In an effort to bring the sometimes astounding results of immunotherapy to more people, Ramalingam is teaming up with renowned immunologist Rafi Ahmed, director of the Emory Vaccine Center.
Ahmed says several factors may explain why immunotherapy drugs, known as checkpoint inhibitors or PD1 blockers, aren’t effective for most lung cancer patients. They work by “waking up” some of a patient’s T cells, an attack arm of the immune system, which have been pushed into dormancy by prolonged contact with cancer. But if there aren’t enough T cells that recognize the tumor to start with, or if the tumor is immunologically not open, that could limit an effective response.

Jennifer Carlisle uses a fluorescence-activated cell sorting (FACS) machine to separate out the T cells that are responsive to checkpoint inhibitor immunotherapy drugs.
“We are trying to overcome this by using combination therapy,” Ahmed says. “We use PD1 blockade to release the brakes on the T cells, but then provide other factors so that the tumor microenvironment is receptive.”
Several years ago, Ahmed’s lab had observed that certain drugs (paradoxically used as immunosuppressants) actually stimulate the long-lived “stem-like” T cells responsible for immunological memory in animal models. The new SPORE will allow researchers to test the effects of combining those drugs with checkpoint inhibitors for a limited time in lung cancer patients before surgery.
Working with Ahmed, Ramalingam and colleagues have already shown that patients whose T cells display a strong proliferative response to checkpoint inhibitors do well later. Recent research suggests that the responsive T cells aren’t in the tumor already, but are waiting elsewhere in the body.
“We need to know more about where the stem-like T cells live, and how they respond to immunotherapy,” Ramalingam says. “The labeling experiments that we plan to conduct as part of the SPORE will be highly informative.”

Researchers in Rafi Ahmed’s lab, Sakshi Malik (left) and Don McGuire, Jr. (right) are looking for patterns of gene activity in T cells.
Scientists are planning innovative experiments, using “heavy water” non-radioactive labeling, which will help them track the T cells that checkpoint inhibitors unleash. Through the SPORE’s multidisciplinary approach, Ahmed says, “We can begin to answer some of lung cancer’s most pressing questions and generate new ones that each of us, working alone, might never have thought to ask.”
by Quinn Eastman • editor Catherine Williams • photos by Jack Kearse • design by Stanis Kodman

Jennifer Carlisle uses a fluorescence-activated cell sorting (FACS) machine to separate out the T cells that are responsive to checkpoint inhibitor immunotherapy drugs.
Jennifer Carlisle uses a fluorescence-activated cell sorting (FACS) machine to separate out the T cells that are responsive to checkpoint inhibitor immunotherapy drugs.

Researchers in Rafi Ahmed’s lab, Sakshi Malik (left) and Don McGuire, Jr. (right) are looking for patterns of gene activity in T cells.
Researchers in Rafi Ahmed’s lab, Sakshi Malik (left) and Don McGuire, Jr. (right) are looking for patterns of gene activity in T cells.

Want to know more? Please visit
Winship Cancer Institute • Emory News Center • Emory University