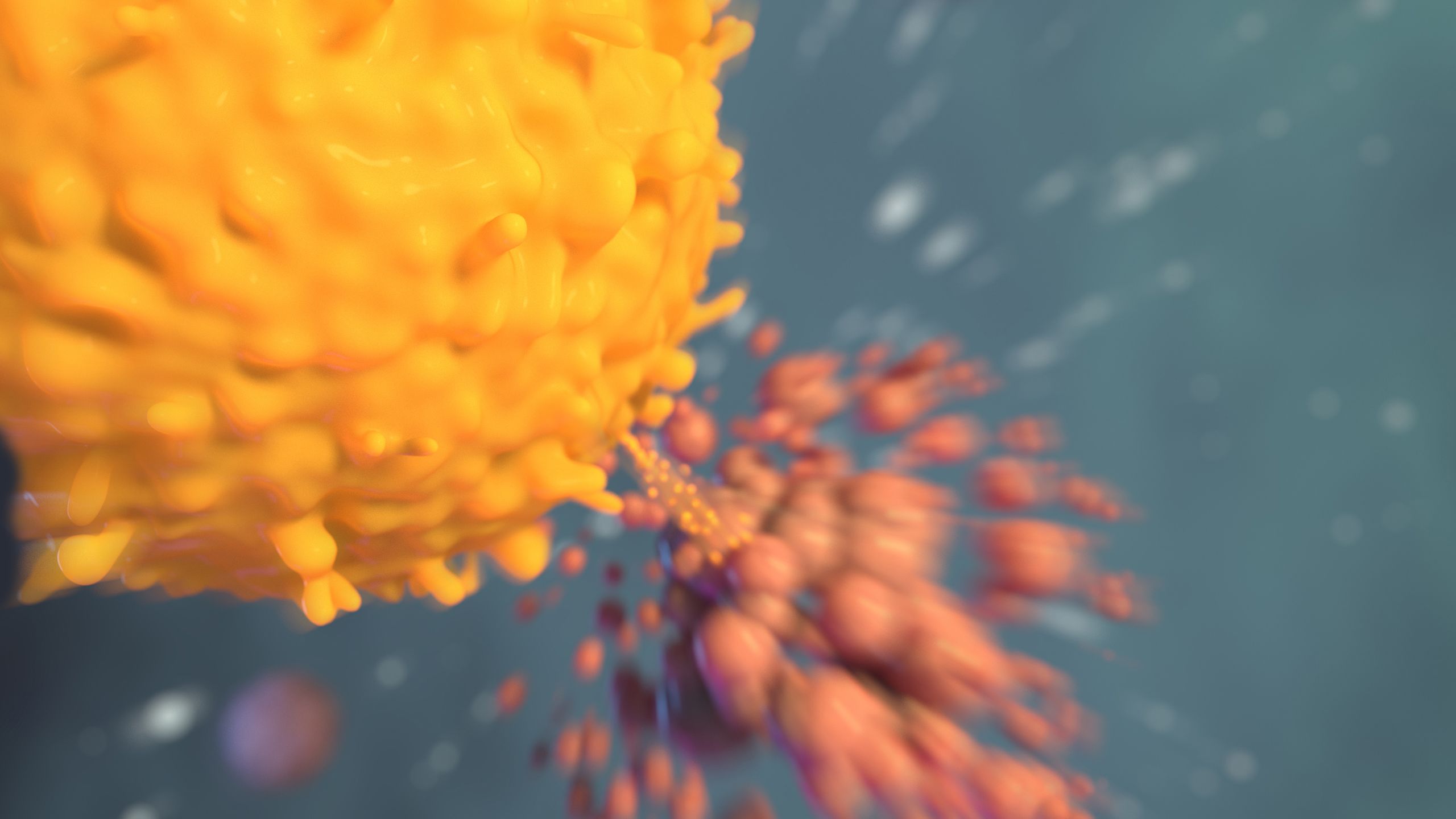
CELL WARS
Turbocharging the Immune System
As troubling as his digestive and upper respiratory symptoms were, she told him, “That lump on your neck is what you should be worried about.”

In early 2016, Ricardo Parks had been experiencing stomach aches and unexplained vomiting spells for a few months. A local doctor prescribed tablets for an upset stomach, but by March, things had gotten even worse. “Anything I ate, I threw up,” he says.

Ricardo Parks (above with his 9-year-old daughter, Recayna) is responding well to immunotherapy treatment for his cancer through a clinical trial.
He lost weight, developed a sore throat, and lost his voice. Parks decided to take action. He enrolled in a health insurance marketplace plan just before the deadline, called around to see what providers took his new insurance, and—mostly by chance—found himself in an Emory GI clinic.
Physician assistant Susan Rubio noticed a swollen lymph node on Parks’ neck from across the exam room. As troubling as his digestive and upper respiratory symptoms were, she told him, “That lump on your neck is what you should be worried about.” Enlarged lymph nodes are often a telltale sign that something is wrong.
Parks lives in Snellville, Georgia, but grew up in the Caribbean, where he says there’s a home remedy for almost every ailment. “In the Caribbean, we get bumps all the time!” he says. “You get a bump, you just brush it off and keep going.” Rubio ordered a biopsy of his lymph node and within a week, the results came back: it was stage IV cancer. Parks was stunned. “I cried,” he says, “everybody [in my family] cried. But by day two, I was like, okay, I got sick. What are we going to do about it?”
A colonoscopy showed a large tumor in his colon, which needed to be removed as quickly as possible. A genetic test revealed that he had microsatellite instability, which is associated with Lynch syndrome. It meant one of the DNA repair genes he inherited—that typically acts like a “spell check” as DNA replicates and divides—was faulty. This explained his unexpected diagnosis at such a young age.
Under the guidance of Joshua Winer, surgical oncologist at Emory’s Winship Cancer Institute, and Bruce Goldsweig, medical oncologist at Winship, Parks underwent a colostomy procedure to remove the tumor, started chemotherapy, and navigated complications with his chemo port. “I never knew it was going to be that rough,” says Parks. “Chemo, you know, it’s killing everything. It’s like a scattershot.”
Despite the challenges, by November 2016, he was in remission.
Then, in January 2017, Parks went in for a follow-up CT scan and the doctors found another lump. The cancer was back. This time, five months of chemotherapy didn’t work. Despite the grim prognosis, Parks kept up his spirits. “I was lucky, no, I was blessed, to meet the right people,” he says.

Dr. Bassel El-Rayes is professor and vice chair for clinical research in Emory's Department of Hematology and Medical Oncology and John Kauffman Family Professor for Pancreatic Cancer Research and associate director for clinical research at Winship.
Bassel El-Rayes, associate director for clinical research at Winship, who specializes in translational research for gastrointestinal cancers, works at the cutting edge of treatments for patients like Parks.
El-Rayes has seen patients with microsatellite instability respond very well to PD-1 checkpoint inhibitor drugs, which take the brakes off the immune system by targeting a mechanism that cancer cells use to cloak themselves. Blocking the mechanism enables the immune system to recognize and attack the cancer cells.
He invited Parks to join a new clinical trial to determine the effectiveness of using a combination of two checkpoint inhibitor drugs, a PD-1 inhibitor with a LAG-3 inhibitor. “He’s had a very remarkable response,” says El-Rayes, a professor in hematology and medical oncology at Emory School of Medicine.
“So that’s why after a certain point, even if the drug goes away, the immune system is onto the cancer. It’s like ‘I got this. I don’t need you anymore.’ ”
Since he joined the clinical trial, Parks’ cancer has continually improved, with almost no side effects along the way.
Doctors caution that individual response to immunotherapy treatments vary, with checkpoint inhibitors being so effective in only a small subgroup of GI patients. However, the response in Parks’ subgroup—those with high microsatellite instability colorectal tumors—is about 50 percent.
Parks is delighted with his results. “It’s the best thing ever,” he says. “When I asked Dr. El-Rayes how long the clinical trial is normally, he said, ‘Two years.’ At that time, I wasn’t thinking I had two years [to live], so even that was good news. I’m so happy to be on this trial, I’ll do it for 10 years if they want me to.”
With chemotherapy or targeted drugs, the doctor relies on the medication to produce the effect and the patient is “sort of like a bystander—the drug is working on the tumor,” says El-Rayes.
With immunotherapy, the patient’s own immune system is fighting the cancer. “So that’s why after a certain point, even if the drug goes away, the immune system is onto the cancer,” El-Rayes says. “It’s like, ‘I got this. I don’t need you anymore.’ ”
These benefits aren’t uniform across all types of cancer, and substantial long-term
studies have yet to be conducted. But for now, the advances in cancer treatment are significant, especially for patients like Parks. “What is unique about the outcomes we’re seeing with immunotherapy [is] the sustainability of the benefit,” says El-Rayes. “It’s very exciting to see that we have patients who are two, three, sometimes four years out—their cancers are still under control and they’ve only used immunotherapy.”
As of now, Parks has no evidence of disease. “There’s a good chance it won’t be active again,” El-Rayes says.
Parks is back to enjoying family time with his wife, Taniesha Blake, and 9-year-old daughter, Recayna. “I want people to know not to give up,” he says.

Ricardo Parks (above with his 9-year-old daughter, Recayna) is responding well to immunotherapy treatment for his cancer through a clinical trial.
Ricardo Parks (above with his 9-year-old daughter, Recayna) is responding well to immunotherapy treatment for his cancer through a clinical trial.

Dr. Bassel El-Rayes is professor and vice chair for clinical research in Emory's Department of Hematology and Medical Oncology and John Kauffman Family Professor for Pancreatic Cancer Research and associate director for clinical research at Winship.
Dr. Bassel El-Rayes is professor and vice chair for clinical research in Emory's Department of Hematology and Medical Oncology and John Kauffman Family Professor for Pancreatic Cancer Research and associate director for clinical research at Winship.
“The chemo didn’t seem to bother me that much, not that I noticed,” he says, “but the radiation, oh my gosh.”


Steven Hatfield and his daughter, Amy Sturgeon, are back to enjoying outings in their camper after his immunotherapy treatment.
Steven Hatfield and his daughter, Amy Sturgeon, are back to enjoying outings in their camper after his immunotherapy treatment.

Dr. Nabil Saba is a professor in Emory's departments of Hematology and Medical Oncology and Otolaryngology, and director of the Head and Neck Medical Oncology Program at Winship.
Dr. Nabil Saba is a professor in Emory's departments of Hematology and Medical Oncology and Otolaryngology, and director of the Head and Neck Medical Oncology Program at Winship.
Combination Therapy
The first PD-1 checkpoint inhibitor drugs were game-changers for advanced lung cancer and melanoma patients, but a significant portion of cancer patients do not respond to them.
One of the next steps was to combine them with other therapies that could enhance their effectiveness.
Steven Hatfield’s doctors decided to try this tactic on his cancer, after other options had failed. Hatfield’s health had taken several twists and turns over the years. At a routine medical exam at his aluminum smelting plant job in the 1990s, he discovered he had diabetes. A decade later, he underwent gastric bypass surgery, lost 200 pounds, and kept the weight off. He’s had a pulmonary embolism, partial thyroid removal, a hernia, and other ailments.
So, when he visited his endocrinologist for a routine check-up, he wasn’t too alarmed when the doctor told him he ought to get the knot on his neck checked out. He waited a few months before getting it examined.
In late 2015, a biopsy determined that the lump was cancerous: stage IV oropharyngeal cancer. Just as he was about to start chemotherapy and radiation, a bout of painful stomach cramps landed him in the hospital. Hatfield had a leak in his small intestine that required a colostomy procedure.
He began chemotherapy and radiation on a tumor at the base of his tongue in February 2016, as soon as he healed from the previous surgery. “The chemo didn’t seem to bother me that much, not that I noticed,” he says, “but the radiation, oh my gosh.”
By the end of six weeks of treatment, Hatfield found it so painful to swallow that he stopped eating. He became weak, dehydrated, and feverish, developing pneumonia. By March 2016, however, there was no cancer seen on his scans. “They couldn’t find it anywhere,” he says.
A year went by. Then, in August 2017, the cancer came back. This time, it had spread throughout his chest and was dangerously close to his heart and lungs, which meant that surgery and radiation were out of the question.
Hatfield met with Nabil Saba, the Winship medical oncologist who oversaw his initial chemotherapy. Saba is an expert in the treatment of head and neck cancer.
Not long before Hatfield’s diagnosis, several checkpoint inhibitor drugs had been approved for use in head and neck cancer by the FDA, after a series of clinical trials in which Emory was a participant.
Patients who receive nivolumab [PD-1 inhibitors] have double the chance of being alive one year after the start of treatment, compared with patients on chemotherapy, says Saba. “Not only that, but they basically also have preservation of their quality of life, whereas the patients who receive standard chemotherapy have deterioration of their quality of life.”
Saba says head and neck cancer is among the toughest to handle for patients, due to symptoms of the disease itself, the toxicity of treatment, scarring, and the proximity to vital structures. “I think immunotherapy stands to make a major impact in head and neck cancer, possibly more so than other tumors,” he says.
At this point, Hatfield’s cancer was considered to be metastatic, incurable stage IV. But his cancer also happened to be the perfect candidate for one of the clinical trials that Saba was working on, testing the effectiveness of a PD-1 inhibitor in combination with a CTLA-4 inhibitor.

Dr. Nabil Saba is a professor in Emory's departments of Hematology and Medical Oncology and Otolaryngology, and director of the Head and Neck Medical Oncology Program at Winship.
“I would say that we’ve entered the era of combination therapy,” says Saba. “We’re past the single agent approvals, and now we’re in a phase where we are exploring ways of banking on this success that we’ve had with single agent checkpoint inhibitors.”
Even though Hatfield was an ideal candidate for the study, Saba says, there was no guarantee that he’d receive the immunotherapy treatment. These trials are randomized, with some patients receiving immunotherapy and some receiving chemotherapy, to compare the differences.
Hatfield was intimidated by the pages of possible side effects on the consent form. “I felt like I didn’t have a choice, you know,” he says. “I really felt like it could be a positive note for me. And if not, well then, I wasn’t afraid of death.”
In August 2017, Hatfield began the clinical trial and was selected for the immunotherapy arm. He receives one drug every two weeks, and a second drug every six weeks, undergoing routine scans to check the progression of his tumor along the way.
His tumor has gone down and stayed down. And he hasn’t experienced any side effects from the immunotherapy. “I apologize to the nurses and doctors all the time, saying, I’m sorry I don’t have any side effects to report!” he laughs. “It’s unbelievable how well it’s worked.”
“What we used to think of as impossible to cure may be within reach of curability with immunotherapy.”
“I feel very fortunate, very lucky,” Hatfield says. He spends time between treatments going camping with his family. “I’ve got three grown children, and they’ve been the biggest part of my life,” he says. Looking ahead, Hatfield will stay on the clinical trial for up to two years, at which point Saba will evaluate his progress and determine if immunotherapy is still a good fit. Clinical trials, both at Emory and internationally, are showing promising results in favor of immunotherapy as a first-line treatment for head and neck cancers.
Saba, like other oncologists, has high hopes. “What we used to think of as impossible to cure may be within reach of curability with immunotherapy,” he says.
“I lost a few days,” she says. “My kids came to visit and said, ‘Who am I? You didn’t know yesterday.’ ”

T Cells to the Rescue
Marylee VanKeuren, a 64-year-old nurse at Emory University Hospital Midtown and a clinical nursing instructor at Emory’s School of Nursing, knew there was something wrong when she started bruising easily without bumping into anything. She was also short of breath, even without exertion. “Just doing normal things,” she says.

After CAR-T therapy, Marylee VanKeuren is looking forward to finally taking a twice-canceled cruise.
When she had a screening mammogram, her lymph nodes were so large you could see them on the scan. The doctor did a biopsy.
Despite her fears, she was pretty sure it would turn out to be nothing. “When you take care of other people, you don’t think anything can happen to you,” she says.
But her diagnosis came back as diffuse large B-cell lymphoma, the most common subtype of non-Hodgkin lymphoma. After a course of recommended chemotherapy, she had a good scan. But a scan two months later was not as good, and more chemotherapy was required.
She had an autologous stem cell transplant, using her own stem cells. During follow-up testing at 100 days, the lymph nodes in her neck showed up as visibly enlarged—so much so that people at her work and church noticed them. “That’s when my doctors started talking about immunotherapy,” she says. “You had to have two previous failed therapies. You don’t just jump right in to CAR-T.”
CAR-T stands for chimeric antigen receptor T-cell therapy, which uses the patient’s own, modified T-cells to attack their specific cancer.

Dr. Jonathon Cohen is an assistant professor in Emory's Department of Hematology and Medical Oncology and medical director of infusion services for Winship.
“Mrs. VanKeuren had an aggressive cancer from the beginning,” says Jonathon Cohen, medical oncologist at Winship and assistant professor of medicine at Emory. “Until now, autologous stem cell transplants represented the best chance at a cure for patients with relapsed non-Hodgkin lymphoma. Unfortunately, she had progressive disease again despite the transplant, and this made her a candidate for the CAR-T treatment. This was particularly exciting as only a few years ago we would have not had such encouraging treatment options for her.”
“It is as if we are training the immune system to identify the cancer and attack it.”
For CAR-T therapy, T-cells are obtained from a patient by collecting their blood through apheresis. The cells are then engineered to recognize the patient’s cancer. “They are infused back into the patient where they go to work, hopefully targeting the patient’s cancer cells,” Cohen says. “It’s as if we’re training the immune system to identify the cancer and attack it.”
Winship has a dedicated cell therapy program, with several physicians who have extensive experience in administering CAR-T therapy. “We’re using it more frequently with each passing month as patients become aware of its potential benefits,” Cohen says. “At this time, it’s only available for patients whose disease has progressed after more traditional therapy, but Emory is one of several centers investigating the use of CAR-T earlier in the disease course, as well as in other lymphoma subtypes and cancers.”
It was a stressful time for VanKeuren—her 88-year-old father had just died, and she was facing another cycle of chemo to make her body less likely to fight the CAR-T cells. “You think you have empathy with your patients, but now I really, really do,” she says.
After the transfusion, she had two of three serious possible side effects of CAR-T: low blood pressure with an elevated heart rate and neurotoxicity, which temporarily affected her cognitive abilities and memory. “I lost a few days,” she says. “My kids came to visit and said, ‘Who am I? You didn’t know yesterday.’ ”
Patients getting immunotherapy sometimes experience side effects due to the “cytokine release”—an inflammatory response caused by the surge of immune cells and their activating compounds (cytokines). “Patients must be monitored closely while they are in the hospital and also in the first few weeks after they are out,” says Cohen. “Fortunately, we are getting better at foreseeing these toxicities and managing them.”
After the initial shock, VanKeuren’s body got to work, fighting the cancer with her turbocharged immune system.
A scan just a few weeks later showed no enlarged lymph nodes, and nothing lighting up, for the first time in years. “It was always just incremental improvements before,” she says. “They gave me a copy of the results, and I had to keep reading it. It was hard to believe after all that time.”
“Mrs. VanKeuren is doing great and has had a complete remission,” says Cohen. “She is several months out from the treatment, and we will be checking on her regularly to ensure she remains in remission.”
Patients without any evidence of disease at the six-month mark have tended to stay in remission for a long time, Cohen says, and potentially could be cancer free.
VanKeuren went from feeling fragile and shaky to exercising and doing water aerobics.She and her husband, Gary, are finally taking an Alaskan cruise that they had to cancel twice during her illness.
“I had made plans when things were not going well…” she says. “Now, my plans are different.”
By Kellie Vinal, Illustration by Michael Konomos

After CAR-T therapy, Marylee VanKeuren is looking forward to finally taking a twice-canceled cruise.
After CAR-T therapy, Marylee VanKeuren is looking forward to finally taking a twice-canceled cruise.

Dr. Jonathon Cohen is an assistant professor in Emory's Department of Hematology and Medical Oncology and medical director of infusion services for Winship.
Dr. Jonathon Cohen is an assistant professor in Emory's Department of Hematology and Medical Oncology and medical director of infusion services for Winship.
Want to know more? Please visit
Emory Medicine magazine website, the Emory News Center, Emory University.