The Better to See You With
Medical imaging evolves from shoe store X-rays to cancer-tracking nanoparticles
By Emory Professor of Physics Emeritus Sidney Perkowitz
The first time I had my heart checked by ultrasound at the Emory Clinic, I didn’t know what to expect. The technologist pressed a small device against my chest and moved it around, using a gel-type substance as a conductor, until an image appeared on a computer screen off to the side. Craning my neck, I could just make out a moving shape. It took me a second to realize what I was seeing. It was my own heart, beating even as I watched it—the closest I’ve ever come to an out-of-body moment.
As a physicist, I understand the mechanics of medical imaging. Put simply, images from inside your own body are gathered using sound waves, X-rays, gamma rays, or radio waves and a magnetic field.
These physical probes enable ultrasound, X-ray computed tomography (CT), positron emission tomography (PET), and magnetic resonance imaging (MRI)—medical tools developed by physicists, engineers, and physicians that are now essential to patient care.
Jon Lewin, executive vice president for health affairs at Emory and CEO of Emory Healthcare, is a professor of radiology and imaging sciences and biomedical engineering, and an innovator in the field. What drew Lewin to medical imaging was its trifecta of tech, biomedical science, and patient impact. “When I got involved in the mid-1980s,” he says, “imaging was going from being very static—looking at X-rays, shadows on a screen—to interrogating and visualizing the human body as never before, even during surgery.”
And the level of detail just keeps accelerating, he says. “The kind of information it is possible to learn through current imaging is transforming how medical care is provided across almost every specialty.”
Take, for example, functional brain imaging—“how thoughts are translated into electrical impulses and blood flow,” says Lewin.
Or the advances made possible by interventional radiology. “Minimally invasive, image-guided therapies have changed what used to be major surgery and a week in the hospital into outpatient surgery through a needle hole,” Lewin says. “I had to tell one patient to at least take the weekend to recuperate.”
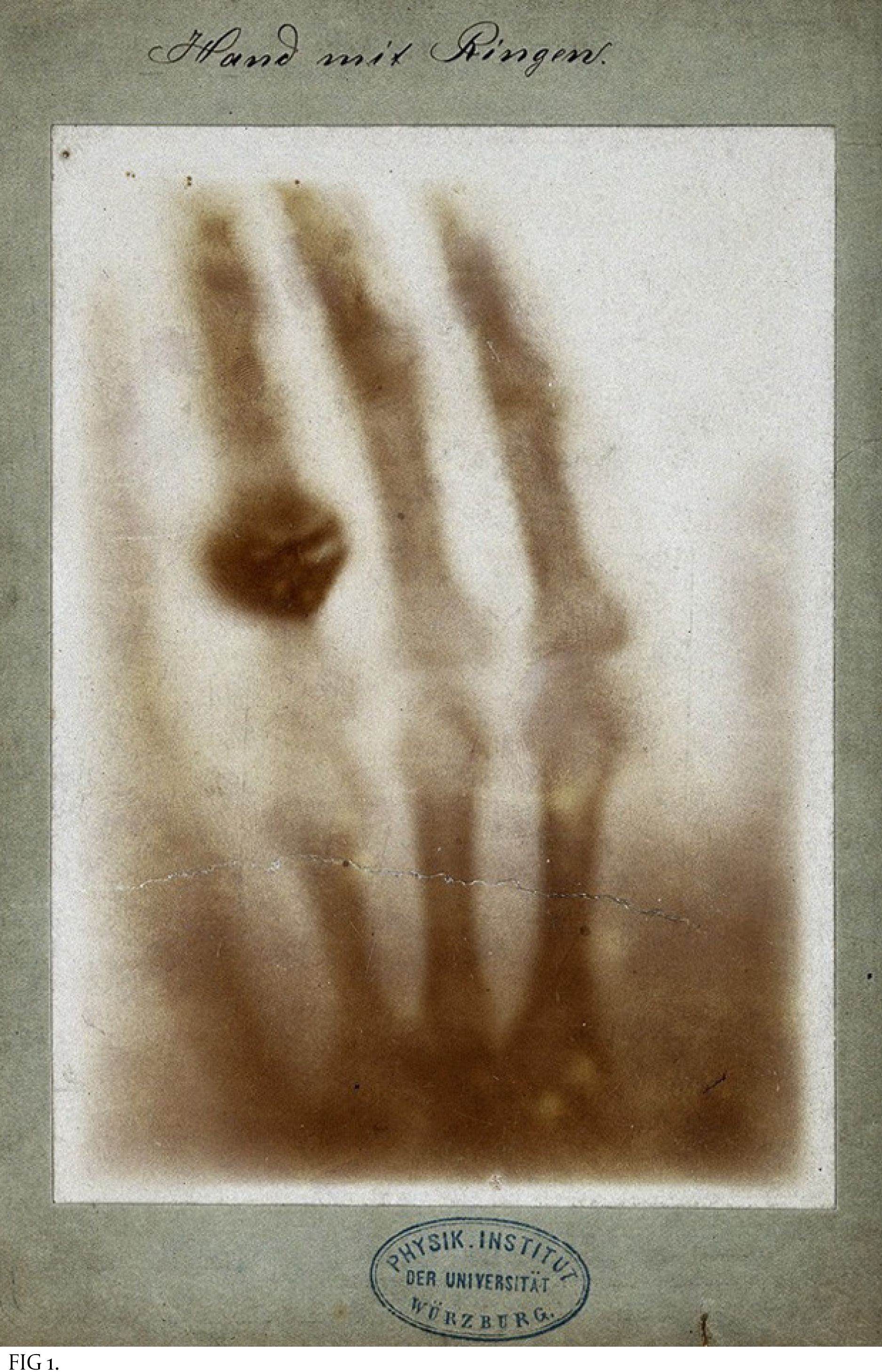
Wilhelm Röntgen's X-ray of the bones in his wife’s hand (which shows her wedding ring on her fourth finger).
THE DARK AGES
Long before these techniques existed, physicians had to try to heal patients without knowing much about what was inside their bodies.
Anatomists and physicians eventually learned more through invasive surgery, by dissecting dead animals and people, and by using tools like the microscope. But it took an accidental observation in 1895 to allow doctors to finally peer inside the living human body without cutting into it.
German physicist Wilhelm Röntgen was working with a novel lab device, a glass tube pumped free of most of its air, with a metal electrode at each end. When high voltage was applied to the electrodes, a glow appeared between them. That was not new, but something else was.
Though the tube had an opaque covering, Röntgen noticed that whenever it was turned on, a fluorescent screen three meters away would light up. Röntgen had discovered an unknown kind of penetrating radiation he dubbed “X-rays.” They generated universal wonderment.

American physician William Morton published The X-Ray: or, Photography of the Invisible and Its Value in Surgery in September 1896.
Only later did physicists learn that X-rays are energetic electromagnetic waves emitted by electrons streaming within the tube. The medical value of these new rays became immediately clear when Röntgen displayed an X-ray photo of the bones in his wife’s hand [FIG 1]. In less than a year, American physician William Morton published The X-Ray: or, Photography of the Invisible and Its Value in Surgery as a guide to medical use of the technique [FIG 2].

Wilhelm Röntgen's X-ray of the bones in his wife’s hand (which shows her wedding ring on her fourth finger).
Wilhelm Röntgen's X-ray of the bones in his wife’s hand (which shows her wedding ring on her fourth finger).

American physician William Morton published The X-Ray: or, Photography of the Invisible and Its Value in Surgery in September 1896.
American physician William Morton published The X-Ray: or, Photography of the Invisible and Its Value in Surgery in September 1896.
CASE STUDY ONE
A 43-year-old sports reporter had a venous malformation involving the sole of his left foot since childhood. He underwent two surgeries as a child to correct this malformation, but had a recurrence after both. He spent 30 years of his life suffering from chronic foot pain that kept him from being active. Approaching his mid-40s, he started to suffer the consequences of his sedentary life with weight gain and high cholesterol level. He sought help at Emory Orthopaedics, but additional surgery was not an option. He was referred to the Emory interventional MRI program, which uses state-of-the-art MRI technology to provide minimally invasive diagnosis and treatment of challenging medical conditions.
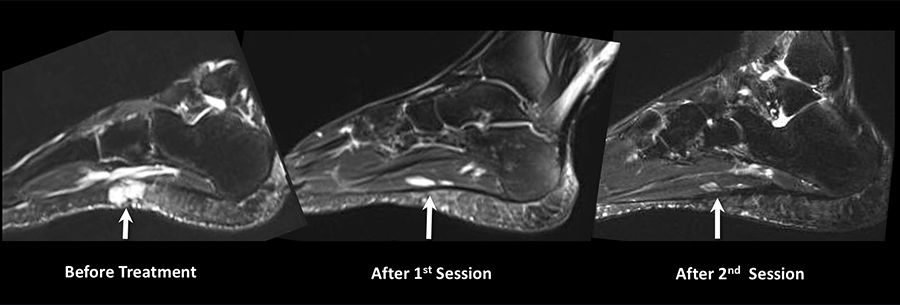
The patient was found to have a scarred sole with a very tender venous malformation. He was given two sessions of percutaneous MRI-guided sclerotherapy, where MRI determines the exact anatomy and extensions of the malformation and then is used for real-time guidance of the procedure. This ensures precise delivery of treatment, reduces the number of sessions and complications, and avoids radiation exposure. The patient responded well, ending up pain free for the first time in 30 years. He is now able to run five to 10 miles a day. “I lost so much weight that people do not recognize me,” he says. Also, his cholesterol level dropped to normal without the need for medications.
—case provided by Dr. Sherif Nour

As late as the 1970s, X-rays were used in shoe stores to gauge how well the shoe fit.
As late as the 1970s, X-rays were used in shoe stores to gauge how well the shoe fit.
CT images of the carotid arteries that carry blood to the head and brain.
CT images of the carotid arteries that carry blood to the head and brain.
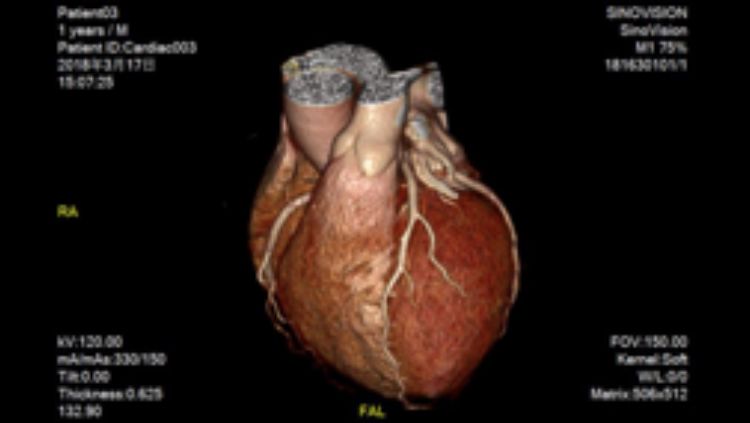
Computer algorithms can help turn CT data into detailed images of organs like the heart.
Computer algorithms can help turn CT data into detailed images of organs like the heart.
FROM 2D to 3D
The early medical use of X-rays did much good but also harm.
With their high energies, X-rays penetrate the soft flesh of the body until they are absorbed by the denser bones, which appear as shadow images in an X-ray photo. Those high energies also can damage DNA and harm living cells. Before this was understood, X-rays were indiscriminately applied. As late as the 1970s, they were used in shoe stores to show a customer’s foot within a shoe [FIG 3].

As late as the 1970s, X-rays were used in shoe stores to gauge how well the shoe fit.
X-rays were also causing cancer in some researchers who worked with them.
Now we carefully limit X-ray dosages for practitioners and patients. Still, the potential harm from X-rays highlights the need for alternative imaging methods with much lower potential for tissue damage.
Computed tomography (CT) creates X-ray images of cross sections of the body that a computer then assembles into a detailed image. When this is done from different angles, changes in density can be converted into pictures of soft tissue, along with bones and blood vessels. CT can diagnose conditions from cancer and cardiovascular disease to spinal problems and trauma.
3D CT Scan
3D CT Scan
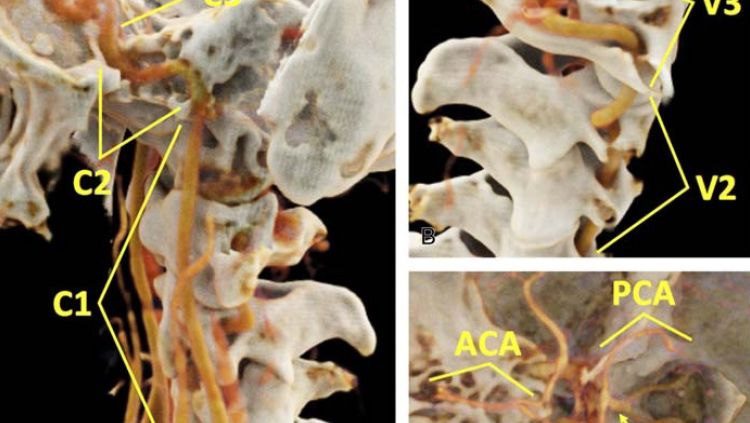
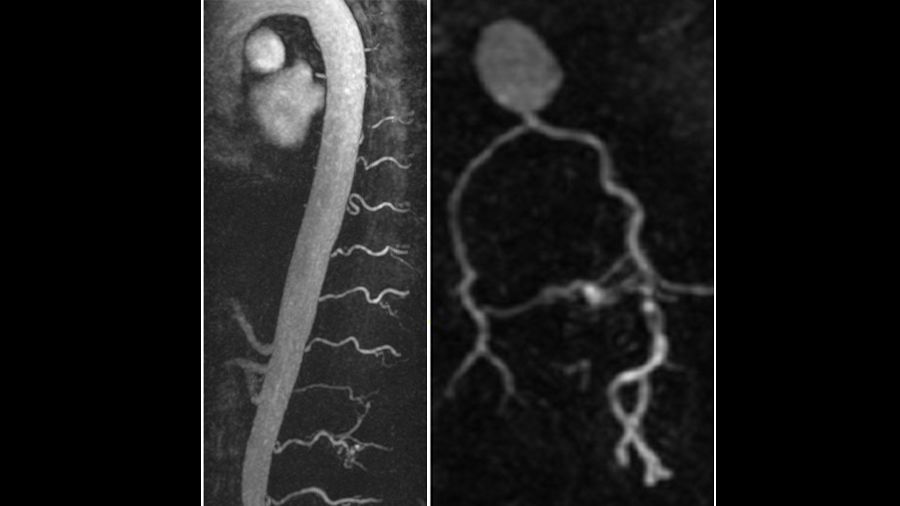
At Emory, biomedical engineer Amir Pourmorteza, assistant professor of radiology and imaging sciences, and colleagues have replaced the usual devices that detect X-rays in CT with more sensitive and selective “photon counting” units. The group’s recent pre-clinical trials on human subjects demonstrate that these detectors enhance images of the carotid arteries that carry blood to the head and brain, [FIG 4] while reducing X-ray dosages by more than 30 percent.

CT images of the carotid arteries that carry blood to the head and brain.
“CT scanners have become lower in radiation, faster, and more precise in two ways: less noise (grainy appearance) and higher image resolution,” Pourmorteza says. “We’re hoping to double resolution.” Think of it like TVs or digital cameras, he says—the same improvement in resolution has taken place in CT scans as well. “You can detect tumors sooner. And you can actually see different colors now, which helps us differentiate diseases,” he says. “It’s like trying to separate an apple from the background leaves—if they both look grey, it can be difficult. But if you can separate the red and green, you can see the apples and the leaves, or the differences between two dense tumors.”
Xiangyang Tang, associate professor of radiology and imaging sciences and director of the Laboratory of Translational Research in CT, works with other X-ray detectors and computer algorithms to turn CT data into detailed images of organs like the heart [FIG 5 ], potentially at lower X-ray exposures.
“Each modality has its own advantages and disadvantages. Our responsibility, as radiologists, is to make sure we do the right thing for the patient,” Tang says. “One of the most exciting progressions in CT is in cardiac imaging. Rapid heartbeats used to cause blurring, but increased scanner speeds mean physicians don’t have to administer beta-blockers to reduce a patient’s heart rate. The new technology can cover the whole heart in one rotation.”
CASE STUDY TWO
A 61-year-old man developed progressive lower back pain with accompanying pain and numbness in his legs. An MRI showed dilated veins in his lower spinal canal that indicated an abnormal connection between arteries and veins called a spinal dural arteriovenous fistula (dAVF). A catheter angiogram evaluating 31 vessels leading to the spinal canal could not find the site of the fistula. A time-resolved MR angiogram (MRA)—an MRI study of the blood vessels—was performed that showed the exact site of the fistula to be within the left L2 segment neural foramen.
A time-resolved MR angiogram (MRA)—an MRI study of the blood vessels—that shows the site of the fistula.
A time-resolved MR angiogram (MRA)—an MRI study of the blood vessels—that shows the site of the fistula.
A repeat catheter angiogram targeting just that vessel confirmed the site of the fistula, and the patient underwent successful surgical treatment of the dAVF. The patient’s symptoms improved following treatment. Since this case, the MRA technique is now used prior to catheter angiography for all suspected spinal dAVFs, which has resulted in more accurate diagnoses and localization of the fistula level, reducing procedure time, radiation dose, and iodinated contrast dose during catheter angiography.
—case provided by Dr. Amit Saindane
MUTUAL ANNIHILATION
Like CT, positron emission tomography (PET) also assembles 2D images into 3D, in this case to locate normal and abnormal metabolic processes in the body.
Positrons are elementary particles first suggested in 1928 when French physicist Paul Dirac predicted that an electron has a kind of fraternal twin, exactly the same but with the opposite (positive) electrical charge. This was confirmed when positrons were found in cosmic rays. Now, of course, we know that every kind of elementary particle has an antiparticle and that antimatter exists along with ordinary matter.
Antimatter is a staple of science fiction because of a dramatic fact: when antimatter meets matter, they annihilate each other in a burst of energy. That burst makes PET the only practical application of antimatter so far.
In PET, the patient is injected with a biologically active and weakly radioactive compound, frequently a special formulation of the sugar glucose. As this is taken up by bodily tissues, it emits positrons, each traveling a millimeter or less before meeting an electron resident in the body. The particles mutually annihilate and generate two gamma rays, powerful electromagnetic waves that, like X-rays, must be carefully monitored. The gamma rays travel in opposite directions until they are detected outside the body, at which point a computer tracks their paths back to their internal point of origin. Adding up many such calculations produces images of regions with strong metabolic activity, as shown by their glucose uptake.
A high metabolic rate typically shows the presence of a tumor, so a main use of PET is to identify and monitor cancers. Brain activity also involves glucose metabolism so PET can diagnose brain tumors, guide brain surgery, and confirm diagnosis of brain deterioration, as in Alzheimer’s disease.
Carolyn Meltzer, chair of radiology at Emory, specializes in PET, and has shown that the best results come when a patient is evaluated by a combined PET/CT scanner—the resulting near-perfect alignment of the two sets of images outperforms separate scans.
For instance, when squamous cell carcinoma, the second-most prevalent type of skin cancer, is suspected in the head and neck, a PET/CT scan facilitates the ability to differentiate between cancer and other abnormalities.
Radiology has medical applications far beyond what people normally think of, says Meltzer. “We are interested in expanding work with imaging agents that have both diagnostic and therapeutic functions, as well as further innovation in image-guided interventional treatments for such wide-ranging conditions as obesity, pain, and tumor ablation.”
ALTERNATIVE TO OPIOIDS
Interventional radiology (IR) is, indeed, a field to watch. Image-guided ablation is an effective treatment for early kidney cancer. Interventional radiologist Sherif Nour also has performed many successful ablations of liver and prostate cancers at Emory. Last year, he traveled to Egypt to teach the technique.
And David Prologo, associate professor of radiology and imaging sciences and an interventional radiologist at Emory Johns Creek Hospital, is helping to develop an IR program in Tanzania. Prologo is also investigating the use of IR in reducing obesity by freezing a portion of the vagus nerve that sends hunger signals to the brain.
David Prologo, associate professor of radiology and imaging sciences and an interventional radiologist at Emory Johns Creek Hospital, talks with a patient.
But much of his and other interventional radiologists’ work is pain related. “We are using our skill set to solve new, long-standing problems, like chronic pain, phantom limb pain, cancer pain. We are able to make dramatic changes in patients’ quality of life,” he says.
One of Prologo’s patients had metastatic lung cancer in her spine and intractable nerve-related pain for more than a year. She underwent CT guided cryoablation of the cancer and the nerve, to block the pain signals. “I ran into her at Publix and she was crying because she was so relieved,” he says.
IR is being used more and more as a drug-free alternative to opioids for pain relief.
“We do our procedure while viewing the CT on a screen, in real time,” Prologo says. “It allows us to guide a needle in safely. We inject the nerve with the anesthetic bupivacaine, a temporary block, and if it works on the patient, we feel justified in doing something more permanent, such as ablation.”
Interventional radiologists work with doctors from all subspecialties— surgeons, ob/gyns, generalists, orthopedists, and bariatric, pain, and palliative care physicians. “We can do more for the patient,” says Prologo, “when we all work together.”
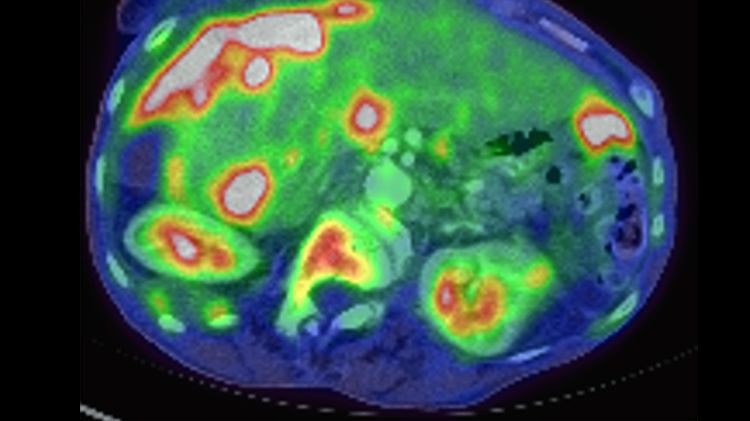
Metastatic ovarian carcinoma, PET CT scan
Metastatic ovarian carcinoma, PET CT scan

Image-guided interventional radiology can be used for tumor ablations and to treat conditions such as obesity and pain, says Carolyn Meltzer, Emory chair of radiology.
Image-guided interventional radiology can be used for tumor ablations and to treat conditions such as obesity and pain, says Carolyn Meltzer, Emory chair of radiology.

David Prologo, associate professor of radiology and imaging sciences and an interventional radiologist at Emory Johns Creek Hospital, talks with a patient.
David Prologo, associate professor of radiology and imaging sciences and an interventional radiologist at Emory Johns Creek Hospital, talks with a patient.
CASE STUDY THREE
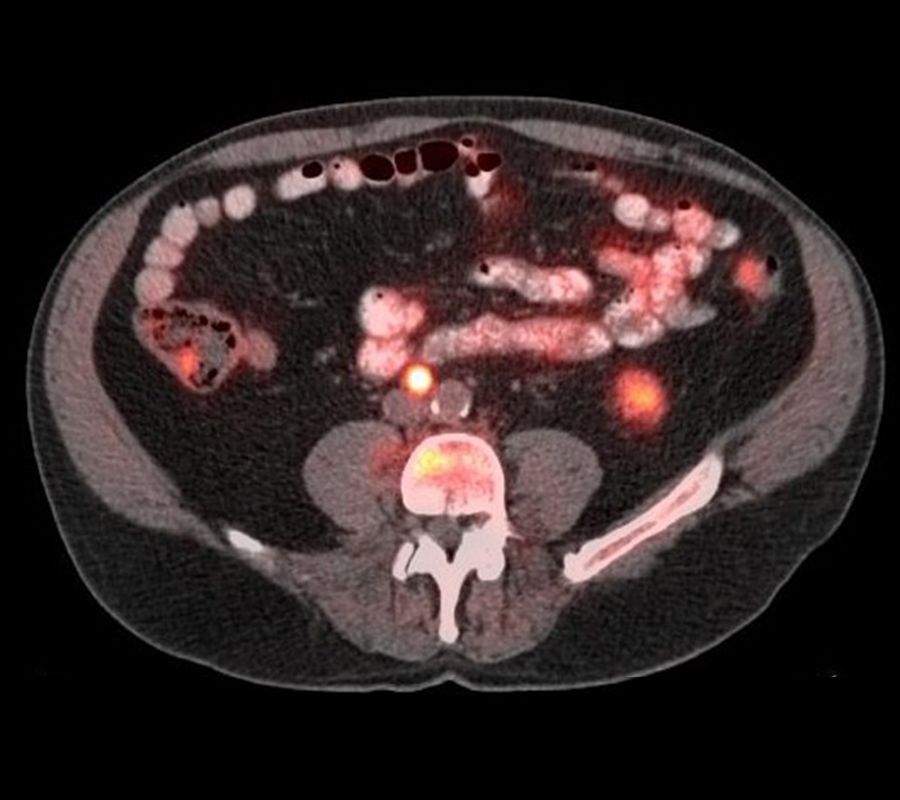
Axumin, a diagnostic imaging agent developed at Emory, shows recurrent cancer in this patient's prostate as well as in lymph nodes outside the pelvis and bone.
Axumin, a diagnostic imaging agent developed at Emory, shows recurrent cancer in this patient's prostate as well as in lymph nodes outside the pelvis and bone.
A man treated for prostate cancer in the past presented with elevated PSA levels. Axumin, a diagnostic imaging agent developed at Emory that can help detect prostate cancer across local and distant sites, showed recurrent cancer in the prostate, but also in lymph nodes outside the pelvis and bone. The ability to determine if a recurrence is in the prostate only, versus outside the prostate, improves the therapy that can be offered to the patient.
—case provided by Dr. David Schuster in nuclear medicine
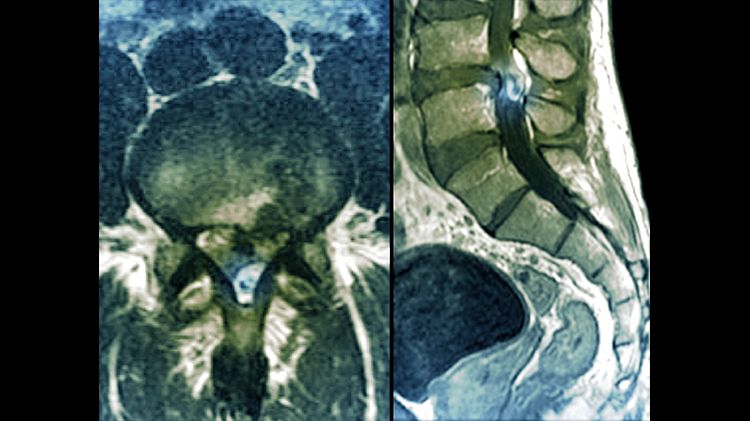
Slipped disc in the lumbar spine, MRI scan
Slipped disc in the lumbar spine, MRI scan
LIKE TINY MAGNETS
The radiation exposure that CT and PET scans require can be an issue for pregnant women or for patients who require frequent scans for diagnosis or follow-up. That’s where ultrasound and magnetic resonance imaging (MRI) come in.
MRI grew out of nuclear magnetic resonance, invented in 1939 by the American physicist Isidor Rabi to study atomic nuclei.

Slipped disc in the lumbar spine, MRI scan
Like tiny magnets, nuclei placed in a strong magnetic field align themselves in specific configurations with different energies. When excited by radio waves, the nuclei emit their own electromagnetic waves at frequencies corresponding to their energies, which act as markers for the particular type of nucleus.
This evolved into the biomedical MRI, which exposes the patient to electromagnetic radiation in the form of radio waves that are too weak to harm cells. The scan also requires a magnetic field thousands of times stronger than the Earth’s. This normally does no harm, although MRIs cannot be used on patients with metallic implants, such as pacemakers.
MRI is a versatile tool for diagnosis and treatment of the neural, cardiovascular, musculoskeletal, and GI systems.
For example, two different MRI studies of heart patients, led by John Oshinski, interim director of the Emory Center for Systems Imaging, showed how to best place the electrical lead from a pacemaker into the wall of a heart to control its beating, and evaluated the effectiveness of a technique to treat rapid, irregular heartbeats.
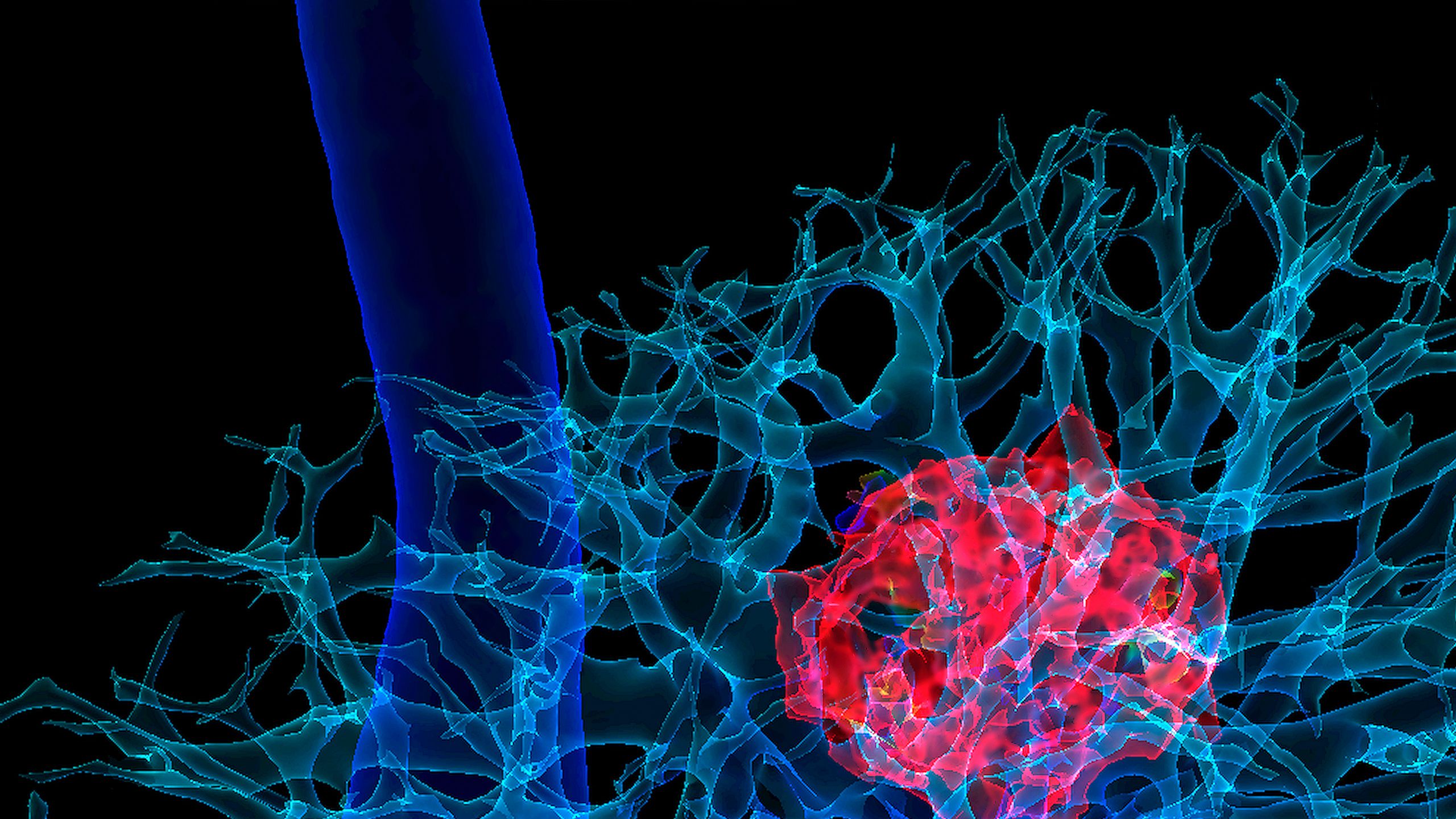
MRI can also assist in taking research from bench to bedside: for example, the use of stem cells and nanomedicine. In 2016, Oshinski, an associate professor in radiology and imaging sciences, and co-researchers used MRI to track stem cells injected into the spinal cords of pigs. The cells were tagged with nanometer-sized iron oxide particles that interacted with the magnetic field during the MRI. They appeared in the resulting images where they confirmed that the cells were properly placed and continuing to function [FIG 6]. Imaging will be critical to develop therapeutic methods like this for the central nervous system.
Magnetic nanoparticles also played roles in recent MRI investigations by Hui Mao, professor of radiology and imaging sciences, showing how nanoparticles carry drugs into tumors and other targeted locations.
CASE STUDY FOUR
A female patient came to us after her son expressed concern for her health because she was obese. She proceeded to lose more than 200 pounds and had plastic surgery to remove excess skin. That surgery was performed elsewhere. She awoke to what felt like a knife in her abdomen. An ultrasound showed a lesion on her right kidney. She came to Emory for an MRI, which confirmed stage I grade III carcinoma, an aggressive form of cancer. She then went to Emory Radiology, where doctors agreed she was an ideal candidate for laser ablation. The cancer was eradicated, with excellent margins. She went home the same day, and her three-week scan and blood work were clean. Her kidney shows no reduced function. She needed no pain medication after the procedure except ibuprofen.
—case provided by Dr. Sherif Nour
MY HEART WILL GO ON
Medical ultrasound differs from the other techniques in using sound waves, not electromagnetic energy or nuclear processes. It began with development of methods to detect underwater objects after the Titanic hit an iceberg and sank in 1912, and to counter German U-boats during World War I. In response, the French physicist Paul Langevin invented devices to put sound waves into water and register the echoes as the waves are reflected from objects, similar to echolocation as used by dolphins and bats. The result was sonar.
Sonar, in turn, inspired the Scottish gynecologist and obstetrician Ian Donald, who learned about it during his military service. In the 1950s, improvising with industrial ultrasound units that detect flaws in metal, he imaged ovarian cysts in women and published a paper showing the first pictures of a fetus’s head in a pregnant woman. Commercial medical ultrasound units soon followed.
The sound waves used in medical ultrasound have frequencies far beyond the upper limit of human hearing, 20 kilohertz. They are typically in the range 1 to 18 megahertz, with the higher frequencies sensing finer details and the lower values providing deeper penetration into the body. A vibrating transducer—the device the technologist at Emory used to examine my heart—is placed against the body or sometimes in a body cavity to send out ultrasound waves. Like light waves, these are reflected, scattered, refracted, or absorbed as they encounter organs and tissues. The returning echoes are detected, and a computer analyzes the results to form a visual image of what the sound is passing through.
With its lack of damaging radiation, obstetric ultrasound is widely and safely used to examine the fetus in pregnant women, allowing prospective parents to see inside the womb. The portability of ultrasound equipment and its real-time results mean it can quickly image tendons, muscles, joints, blood vessels and organs. For instance, it can be used to examine the size, shape, and pumping capacity of the heart right in the doctor’s office.
THE SOUND AND THE FURY
Successful ultrasound imaging depends on putting sound energy into the body with efficient transducers and obtaining high-contrast images. These can be enhanced by using contrast agents—gas-filled microbubbles that strongly reflect sound waves and improve the contrast when blood vessels are being examined.
Researchers at the Coulter Department of Biomedical Engineering (BME) at Emory and Georgia Tech address both of these issues. Brooks Lindsey, assistant professor of BME, is using Georgia Tech’s clean room facilities and other resources to design better transducers and contrast agents in cooperation with Emory physicians.
And Stanislav Emelianov, professor of electrical and computer engineering and BME and director of the ultrasound imaging and therapeutics research lab, has developed a new laser-based contrast agent. In 2017, his team showed that nanodroplets filled with a specific liquid can be vaporized by laser pulses to form highly reflective, gas-filled microbubbles. These quickly re-condense into liquid bubbles that can be reactivated over multiple cycles. This method produces superior high-contrast images of lymph nodes, which can show the spread of cancer.
“The radiology department provides access to imaging for researchers throughout Emory, not only clinical, but also brain health, public health, and animal research,” says Elizabeth Krupinski, professor and vice chair for research in radiology and imaging sciences. “A lot of key studies in developing biomarkers, contrast agents, and tracers have to be done in animals first.”
The future of imaging science will undoubtedly involve artificial intelligence and big data. But imaging can never completely take the place of good clinical skills, says Laurence Sperling, who directs Emory’s preventive cardiology program. Sperling reminds students and young physicians that effective clinical care requires “listening to, talking to, and touching the patient. We are taking care of people, not pictures of people.”
By Sidney Perkowitz, emeritus professor of physics.
Want to know more? Please visit Emory Medicine magazine website, the Emory News Center, Emory University.