Researchers have identified antibodies that may be able to defend people against multiple species of the Ebola virus family. The antibodies were derived from two individuals who survived Ebola virus disease and were treated at Emory University Hospital in 2014.
The findings were published in Cell, following a collaboration between the lab of Rafi Ahmed, PhD, at Emory and scientists at La Jolla Institute for Immunology, led by president and CEO Erica Ollman Saphire, and Gabriella Worwa at the National Institute of Allergy and Infectious Diseases (NIAID).
The results suggest that if deployed as emergency antiviral drugs, the antibodies have distinctive properties that could save lives in future Ebola outbreaks. A combination of two of the antibodies can rescue infected monkeys from otherwise deadly infections, when administered days after exposure.
“These antibodies work even if they are given four days after infection has started,” says Ahmed, director of Emory Vaccine Center and a Georgia Research Alliance Eminent Scholar. “It was amazing to see how quickly the virus level comes down after monoclonal antibody therapy.”
The researchers say that these antibodies could be deployed in situations when existing therapies are unlikely to be effective. Existing available antibody drugs are effective when given early after infection, but less so after infection has advanced and systemic viral levels are high.
The antibodies were isolated from blood samples donated by survivors treated at Emory, more than a year after they left the hospital. Scientists think that the long period after the infection had been subdued helped the individuals’ immune systems to refine their weapons against the virus.
“We think that the extended maturation period was important for the generation of these antibodies,” says Carl Davis, MD/PhD, an instructor in Ahmed’s laboratory and co-first author of the paper. “We did not see antibodies that had these properties early on, after the patients left the hospital.”
Beginning with samples from the survivors, Davis searched for immune cells that produced antibodies that had the ability to bind two different strains of the Ebola virus: Zaire and Sudan. Cells producing antibodies that recognized the Zaire strain were abundant, but the task of identifying cells that recognized both strains was more like finding a golden needle in a haystack, he says.
Countermeasures and vaccines have been developed and tested against the Zaire strain, but not against the Sudan strain. Clinical trials such as the 2018-2019 PALM study have established a standard of care for the Zaire strain of Ebola.
“Finding antibodies with this breadth is important because we don't know which virus in the genus of ebolaviruses is going to break out next,” says Saphire, PhD, co-senior author of the Cell paper.
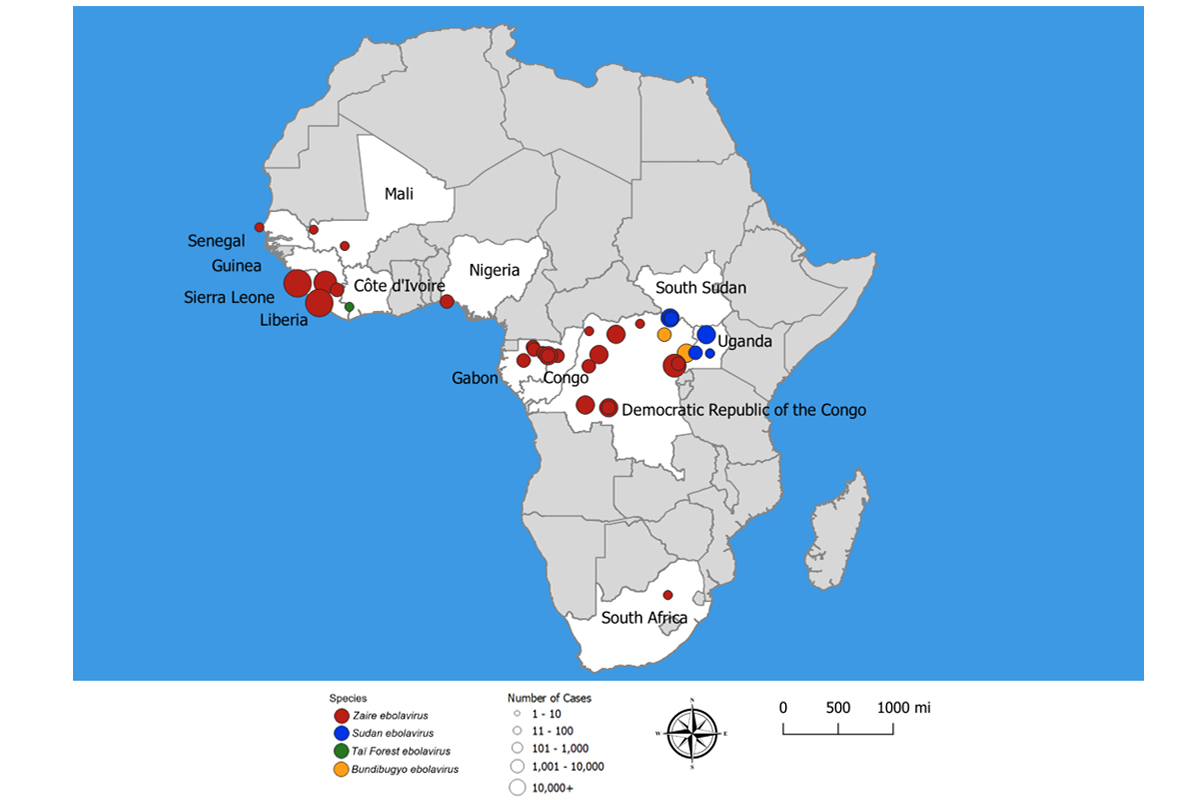
Ebola virus disease outbreaks have killed thousands of people, mostly in West Africa. The most recent outbreak, in the Democratic Republic of Congo, ended in 2021.
Centers for Disease Control and Prevention
Distinctive binding pattern
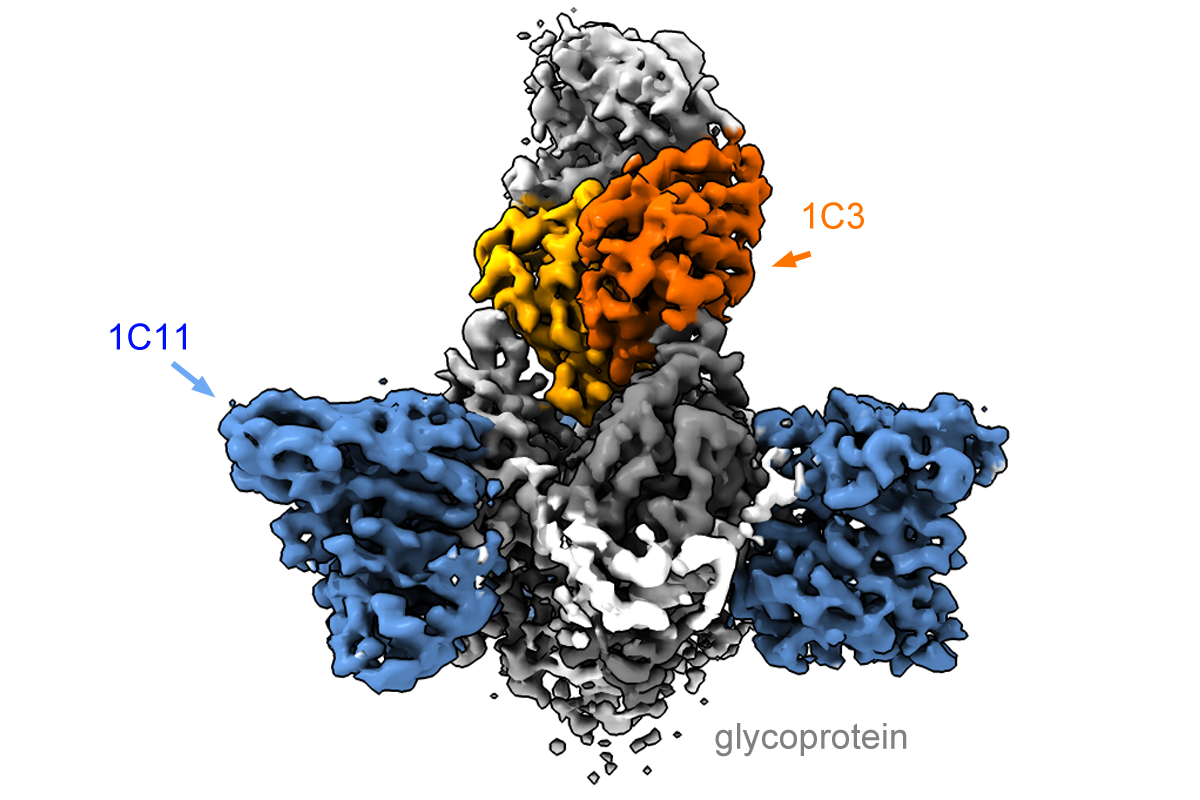
Using cryo-electron microscopy, Saphire’s laboratory obtained detailed images of how the two antibodies attach to the viral glycoprotein. Binding to itself in symmetrical arrangements of three (trimers), the glycoprotein forms the surface of the virus and the virus uses it to enter host cells.
Based on where they bind the glycoprotein as well as their potency in fighting infections, the new antibodies may be able to circumvent a mechanism the virus uses to subvert the immune response. One antibody binds the trimer form of the glycoprotein in an asymmetrical mode, and does not bind to a singular soluble form produced in abundance by the virus as a decoy.
“The antibody [called 1C3] is able to block three sites on the virus at the same time, using different loops and structures to anchor into each one,” Saphire says. “That is remarkable.”
The other antibody binds to the fusion machinery the virus would normally use to enter and infect host cells, which looks similar between Sudan and Zaire. This could give the combination activity across several varieties of the virus.
The biscuit test
Led by co-author Worwa, PhD, from NIAID, researchers tested the antibodies isolated at Emory in monkeys infected with either Zaire or Sudan strains of Ebola. The experiments were conducted with the highest level of safety measures (Biosafety Level 4) at Fort Detrick in Maryland, with antibodies administered intravenously at day 4 and 7 after the monkeys were infected.
The scientists were surprised by the speed at which the antibodies could take effect. Just like humans, when monkeys become sick from infection, they lose their appetite. The monkeys treated with the two antibodies regained their appetite and were willing to eat their standard food (hence, the term “biscuit test”).
Could the strategy of looking for rare cross-reactive antibodies be applied to create better therapies for other viruses, such SARS-CoV-2? With SARS-CoV-2, researchers face a similar problem of trying to anticipate which parts of the coronavirus will change, as viral evolution continues and new variants emerge.
“The immune system has the capacity for this type of response, even if it takes a long time to develop,” Davis says. “The raw material to generate antibodies with these kinds of exquisite specificities is present in everyone.
Along with Davis, the co-first authors of the Cell paper are La Jolla postdoctoral fellows Xiaoying Yu, PhD and Jake Milligan, PhD.
This research was supported by NIAID (U19AI142790, U19AI109762), the Consortium for Immunotherapeutics against Emerging Viral Threats, and DARPA contract W31P4Q-14-1-0010.