How protein assemblies drive cell movement
Scientists uncover startling new insights into the mechanics of cellular motion
Actin, one of the most abundant and versatile proteins in the body, assembles into filaments that are part of the skeleton of living cells, giving them shape. Actin is also essential to the mobility of cells. As actin filaments elongate inside a cell they push against its membrane, enabling the cell to move forward. These same forces generated by the dynamics of actin assemblies allow a stem cell to shape-shift and divide into two daughter cells. Or an immune cell to engulf and kill an invading bacterium.
Biophysicists at Emory University and biochemists at Ohio State University have uncovered a startling new insight into how actin filaments can form and generate cellular movements. Instead of just growing from one end, as scientists have believed for decades, actin filaments can elongate from both ends.
“Our findings upend nearly 40 years of a wrong assumption,” says Shashank Shekhar, Emory assistant professor of physics and cell biology, whose lab’s unique techniques made the discovery possible. “The insight gives us a whole new understanding of the processes underlying how living cells may move.”
The breakthrough has wide implications for research into a range of biological processes, including the hyper mobility that allows cancer cells to metastasize and spread from one part of the body to another and the role of actin in infectious diseases.
Science Advances published the work led by Elena Kudryashova and Dmitri Kudryashov, biochemists at Ohio State University. Co-authors include Shekhar Lab members Ankita (who goes by one name), an Emory graduate student in physics, and Heidi Ulrichs, a graduate student in Emory’s biochemistry, cell and developmental biology program.

From left, biophysicist Shashank Shekhar and graduate students Heidi Ulrich and Ankita, co-authors of the new paper.
From left, biophysicist Shashank Shekhar and graduate students Heidi Ulrich and Ankita, co-authors of the new paper.
It is long established that many bacterial and viral pathogens can hijack control of the actin “motor,” using it to propel themselves, although it is less understood how this hijacking works.
The Kudryashov Lab has a strong track record in uncovering how bacterial toxins influence the host’s actin cytoskeleton.
Cholera is an acute diarrheal illness caused by infection with Vibrio cholerae bacteria. The Kudryashov Lab discovered that toxins secreted by the bacteria caused uncharacteristic changes in the host cell’s actin organization. That led them to want to explore how the toxins might affect actin elongation.
The Ohio State team turned to Shekhar’s biophysics lab at Emory to help investigate.
Pioneering methods in biophysics
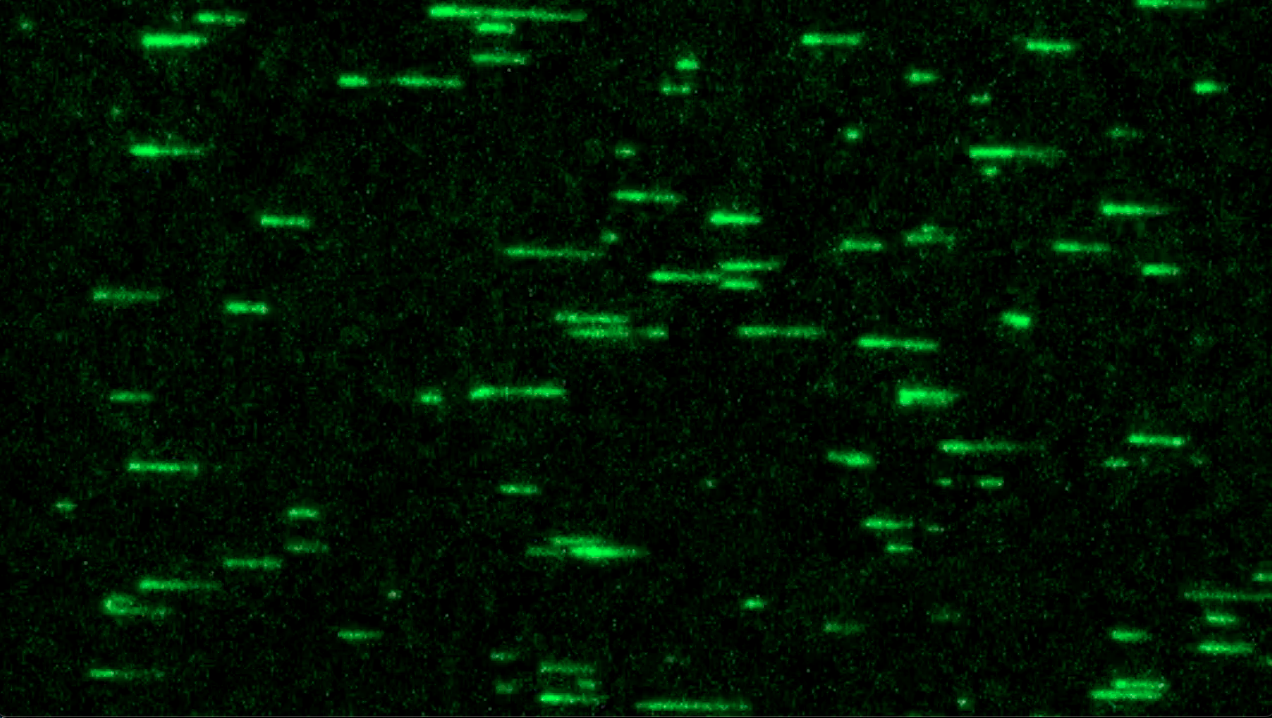
The Shekhar Lab is one of only a handful in the world using the highly specialized technique of microfluidic-assisted total internal reflection fluorescence microscopy (mf-TIRF) for studying the dynamics of how the actin cytoskeleton remodels itself.
“Actin molecules are constantly jiggling and moving around so it’s difficult to do precise measurements on them,” Shekhar explains. “TIRF is a fancy way of saying that we attach fluorescent lights to single protein molecules so that we can better observe what they are doing through a microscope.”
Molecules are introduced into a microfluidic system on a microscope slide. This system allows the researchers to rapidly change the biochemical conditions while simultaneously imaging large numbers of individual actin filaments.
“You need fine control of chemical, biological and physical parameters to set up this technique, which makes it a bit tricky,” Shekhar says.
Video by the Shekhar Lab shows how actin filaments elongate from the "minus" end of the filaments, spurred by the bacterial toxin that causes cholera.
Video by the Shekhar Lab shows how actin filaments elongate from the "minus" end of the filaments, spurred by the bacterial toxin that causes cholera.
For the current paper, the researchers devised a novel experiment to observe the effect of the Vibrio toxin on actin. Instead of actin molecules, they tagged molecules of the toxin and anchored them to a microscope slide. They then allowed untagged, non-fluorescent actin monomers — single units that connect to form filaments — to float on the slide.
Previously, it was thought that actin filaments only grow from their barbed, or “plus” end. A monomer breaks off from the “minus” end of the filament, gets “recharged” with energy and can again be added to the plus end, causing the filament to elongate in that direction. The phenomenon is known as treadmilling.
The Emory team, however, observed for the first time that this treadmill can also run in reverse. In the presence of the Vibrio toxin, actin monomers from the plus end of a filament break off and join to the minus end.
The researchers watched as the fluorescent portion of the actin filament moved away from the anchored toxin, like a curtain sliding, as the untagged actin monomers continued to grow in that direction.
“The discovery turns the whole established concept of how actin filaments can assemble on its head,” Shekhar says. “I think it will reinvigorate this field of study by opening up a whole new realm of questions.”
One key question that the Emory team is now investigating is whether any proteins naturally present in the body may have the same reverse-treadmill effect on actin assembly as the Vibrio toxin.
Story by Carol Clark

