Chemical Catalyst Turns
'Trash' to 'Treasure'
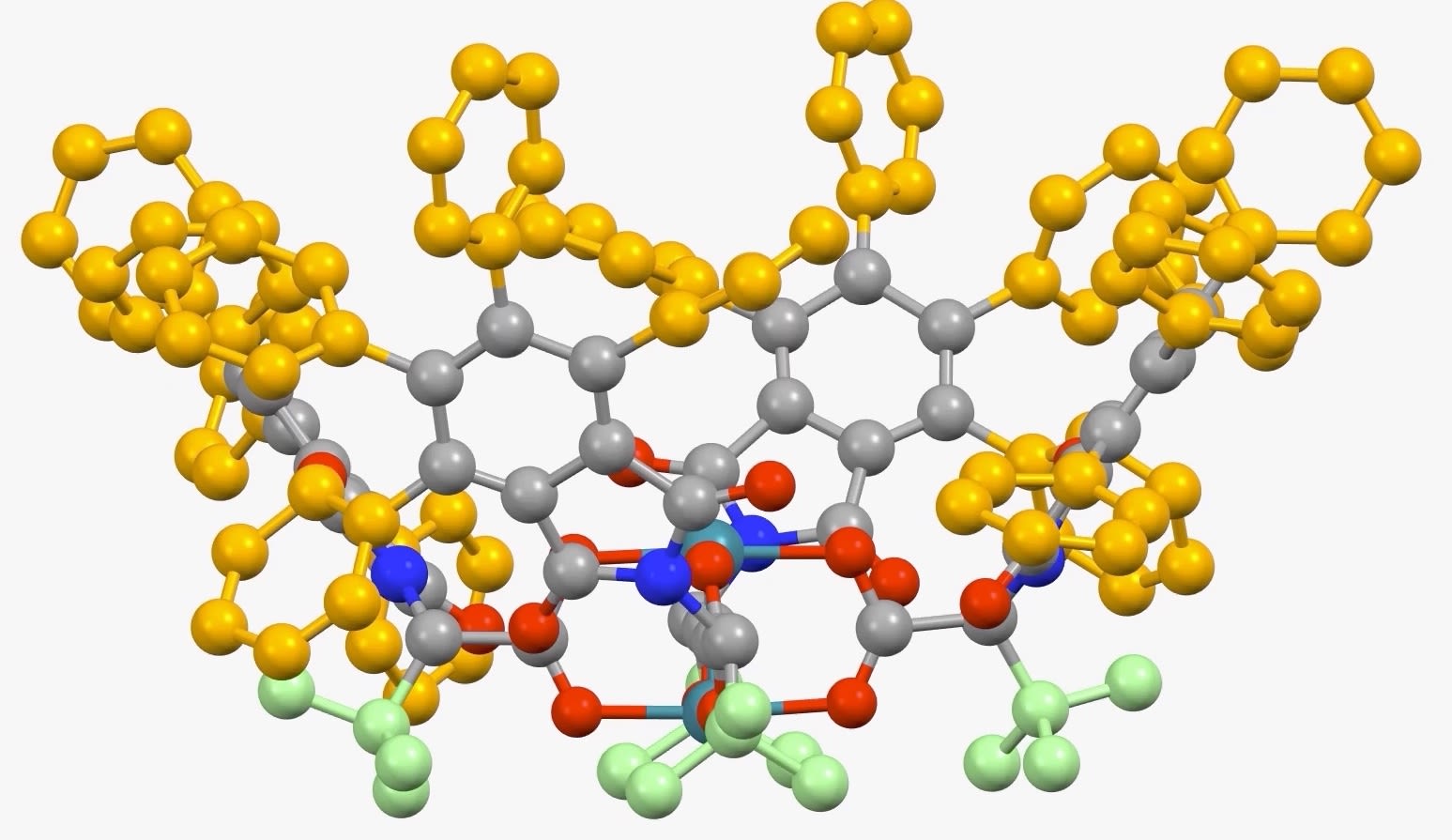
Rotating image (above) shows a 3-D model of the latest dirhodium catalyst developed at Emory University. (Dan Morton, Davies Lab)
For decades, chemists have aspired to do carefully controlled chemistry on carbon-hydrogen bonds. The challenge is staggering. It requires the power of a miniature wrecking ball to break these extremely strong bonds, combined with the finesse of microscopic tweezers to single out specific C-H bonds among the many crowded onto a molecule.
The journal Nature published a method that combines both these factors to make an inert C-H bond reactive — effectively turning chemical “trash” to “treasure.”
“We can change a cheap and abundant hydrocarbon with limited usefulness into a valuable scaffold for developing new compounds — such as pharmaceuticals and other fine chemicals,” says J.T. Fu, a graduate student at Emory University and first author of the paper.
The Nature paper is the latest in a series from Emory University demonstrating the ability to use a dirhodium catalyst to selectively functionalize C-H bonds in a streamlined manner, while also maintaining virtually full control of the three-dimensional shape of the molecules produced.
“This latest catalyst is so selective that it goes cleanly for just one C-H bond — even though there are several C-H bonds very similar to it within the molecule,” says Huw Davies, Emory professor of organic chemistry and senior author of the paper. “That was a huge surprise, even to us.”
This dirhodium catalyst works on a substrate of tert-butyl cyclohexane, a hydrocarbon — one of the simplest of organic molecules, consisting entirely of C-H bonds.
“Not only can we do a totally unprecedented reaction, we can do it under extremely simple conditions,” Davies says. “Tert-butyl cyclohexane is a classic organic structure in chemistry. That helps validate the mainstream potential of C-H functionalization.”
The co-authors of the Nature paper are Djamaladdin Musaev, director of Emory’s Cherry L. Emerson Center for Scientific Computation; Zhi Ren, a post-doctoral fellow in the Davies lab; and John Bacsa, facilities director of Emory’s X-ray Crystallography Center.
Organic synthesis traditionally focuses on modifying reactive, or functional, groups in a molecule. C-H functionalization breaks this rule for how to make compounds: It bypasses the reactive groups and does synthesis at what would normally be considered inert carbon-hydrogen bonds, abundant in organic compounds.
Only a tiny bit of the catalyst is used for a reaction, and most of it can be recycled.

The aim is to efficiently transform simple, abundant molecules — in some cases even chemical waste materials — into much more complex, value-added molecules. Functionalizing C-H bonds opens new chemical pathways for the synthesis of fine chemicals — pathways that are more streamlined, less costly and cleaner.
Organic synthesis, for example, typically involves the use of many reagents, and can produce toxic, inorganic byproducts.
In contrast, each dirhodium catalyst developed by the Davies lab uses only a single reagent and speeds up a reaction without being used up in the reaction. Most of the catalyst can be recycled and the only byproduct generated is nitrogen, which is innocuous.
Chemists experimenting with C-H functionalization often use a directing group — a chemical entity that combines to a catalyst and then directs the catalyst to a particular C-H bond. The process works, but it is cumbersome.
The Davies lab bypassed the need for a directing group by developing catalysts encased within three-dimensional scaffolds. The bowl-shaped scaffold acts like a lock and key to allow only particular C-H bonds in a compound to approach the catalyst and undergo the reaction.
The only byproduct of the reaction is nitrogen, which is innocuous.

"Each of the catalysts are unprecedented, achieving a different kind of selectivity than has been seen before,” Davies says. “We’re developing a toolkit of new catalysts and reagents that will do selective C-H functionalization at different sites on different molecules.”
In addition to controlling site selectivity, the scaffold of the dirhodium catalysts controls the chirality of the molecules produced in the reaction. Chirality, also known as “handedness,” refers to a property of three-dimensional symmetry. Just as the human hand is chiral, because the right hand is a mirror image of the left, molecules can be “right-handed” or “left-handed.”
The handedness of a molecule is important in organic chemistry, since this 3-D shape affects how it interacts with other handed molecules. When developing a new drug, for instance, it is vital to control the chirality of the drug molecules because biological molecules recognize the difference.
Video shows a model of a catalyst developed at Emory in 2016.
The current Nature paper describes the fifth major new catalyst for C-H functionalization that the Davies lab has developed during the past two years.
As a graduate student, Kuangbiao Liao (who has since received his PhD from Emory and is now working for the pharmaceutical company AbbVie) was first author on two papers that appeared in Nature and another published by Nature Chemistry for catalysts developed in 2016 and 2017. Graduate student Wenbin Liu led the work on a fourth catalyst developed earlier this year, published by the Journal of the American Chemical Society.
“We’ve achieved exquisite catalyst control that is beyond what people thought would be possible even two or three years ago,” Davies says. “It’s incredible what my students have been able to achieve.”
The Davies lab is now exploring adding electronic effects to its dirhodium catalysts. “Instead of just interacting with inert shapes, we want our catalysts to have the ability to electronically repel or attract different molecules,” Davies explains. “That could make our methods even more sophisticated and subtle than what we can achieve now, opening up additional new chemical pathways.”
Huw Davies
is used to getting strange reactions

A native of Wales, Huw Davies went to the University of East Anglia, England, where he received his PhD in organic chemistry. It was in New Jersey, however, where he moved for a post-doctoral fellowship at Princeton, that he found his career path.
“I started working with a dirhodium catalyst,” recalls Davies, “and I was impressed with how it did carbene reactions so cleanly. It was incredibly reactive and well-behaved. I decided to focus my career on it because I thought it was going to be a game-changer.”
His gut reaction, combined with his willingness to seek out new methods for doing chemistry, paid off. Davies went on to lead the development of a series of dirhodium catalysts that speed up, simplify and expand the possibilities of organic chemistry, setting the stage for the synthesis of new molecules.
The American Chemical Society recently selected Davies to receive its 2019 Herbert C. Brown Award for Creative Research in Synthetic Methods. Four of the 21 previous winners of the prestigious award have received Nobel Prizes, and all of them are considered superstars in their field.
“It’s a great honor to be included among them,” says Davies, who joined Emory in 2008 as the Asa Griggs Candler Professor of Chemistry.
In 1996, Davies patented his first powerful catalyst made from dirhodium, crystallized into a helix. Rhodium, a member of the platinum group, is one of the rarest and most valuable precious metals. The catalyst, however, generates reactions so efficiently that less than an ounce of the catalytic material could theoretically be used to create a ton of synthesized product. That makes it highly scalable and cost-effective for drug production.

The Davies lab at Emory University.
The Davies lab at Emory.
Shortly after joining Emory, Davies became the founding director of the National Science Foundation’s Center for Selective C-H Functionalization (CCHF), a consortium based at Emory and encompassing 15 major research universities from across the country as well as industrial partners.
Traditionally, organic chemistry has focused on the division between reactive molecular bonds and the inert bonds carbon-carbon (C-C) and carbon-hydrogen (C-H). The inert bonds provide a strong, stable scaffold for performing chemical synthesis with the reactive groups. C-H functionalization flips this model on its head, making C-H bonds reactive.
The CCHF is leading this paradigm shift in the strategy of synthesis, which holds great promise for creating new pathways for drug discovery and the production of new materials to benefit everything from agriculture to electronics.
Watch a video about the formation of the NSF Center for Selective C-H Functionalization
“When you’re developing a new drug, it can be a big advantage not to do it the same way that everyone has for the past 100 years,” Davies says. “New methods can allow you to introduce new structures that lead to new potential drug candidates.”
Collectively, the CCHF has published more than 200 papers and developed dozens of new catalysts for C-H functionalization, including five major new dirhodium catalysts from the Davies lab.
The center is also revolutionizing the way that chemistry is researched and taught, breaking down the walls of individual labs and institutions to form collaborations and student exchanges that reach across the country and across continents.

Kathryn "Katie" Chepiga did research in Japan as an Emory graduate student. She published findings that C-H functionalization speeds synthesis in marine alkaloids from a sea sponge that have shown potential for drug therapies. Chepiga received her PhD in 2018 and now works at Bayer Pharmaceuticals in Germany. (Kay Hinton, Emory Photo/Video)
Kathryn "Katie" Chepiga did research in Japan as an Emory graduate student. She published findings that C-H functionalization speeds synthesis in marine alkaloids from a sea sponge that have shown potential for drug therapies. Chepiga received her PhD in 2018 and now works at Bayer Pharmaceuticals in Germany. (Kay Hinton, Emory Photo/Video)
The NSF Science Across Virtual Institutes (SAVI) program this year awarded another $300,000 to the CCHF to continue its student exchange program, including labs in the United States, South Korea, Japan and Germany. Undergraduates, graduate students, and post-doctoral fellows can participate in national and international exchanges, learning the techniques of other labs while bringing in new ideas of their own.
“We’ve got this incredible collaborative environment where organic chemists aren’t just sharing results —they’re sharing ideas,” Davies says. “That’s rare. And we’ve expanded that environment beyond our network of universities to also engage the pharmaceutical industry.”
The Herbert C. Brown Award is only the latest recognition of Davies’ groundbreaking career in organic synthesis. Other recent accolades for Davies include being named Fellow of the Royal Society of Chemistry (2007), Fellow of the American Chemical Society (2009), Fellow of the American Association for the Advancement of Science (2012), Fellow of the National Academy of Inventors (2015) and receiving the Paul Rylander Award from the Organic Reactions and Catalysis in Synthesis Society (2018).

To learn more
Please visit the Center for Selective C-H Functionalization, the Emory Department of Chemistry, the Huw Davies Lab, the Cherry L. Emerson Center for Scientific Computation, and the X-ray Crystallography Center.